Guide to Sphingolipid: Structure, Classification, and Detection Methods
Sphingolipids are a diverse and vital class of lipids that play crucial roles in cellular processes and signaling. These complex molecules are integral to maintaining cell membrane integrity and are involved in a range of biological functions, from apoptosis and cell adhesion to immune responses and signal transduction. This comprehensive guide delves into the world of sphingolipids, covering their structure, biosynthesis, catabolism, and the methods used for their extraction and detection. Whether you're a researcher, student, or industry professional, this article provides valuable insights into the fascinating realm of sphingolipids and their significant impact on health and disease.
1.What is Sphingolipid?
Sphingolipids are a fascinating class of lipids, essential for various cellular processes. Derived from a backbone structure known as the "sphingoid base," which is a long-chain amino alcohol, sphingolipids play critical roles in cell structure and signaling. The most common sphingoid base is sphingosine, but other variations such as dihydrosphingosine and phytosphingosine also exist. These backbones distinctly set sphingolipids apart from other lipid classes like glycerophospholipids and cholesterol derivatives.
The classification of sphingolipids can be quite complex due to the presence of various functional groups attached to the sphingoid base. The major classes include ceramides, sphingomyelins, cerebrosides, gangliosides, and sphingosine-1-phosphate. Each of these classes boasts unique structural and functional attributes, making them indispensable in numerous cellular functions.
- Ceramides are the simplest form of sphingolipids, consisting of a sphingoid base linked to a single fatty acid through an amide bond. These molecules are not only precursors for more complex sphingolipids but also play significant roles in cellular signaling pathways, particularly those related to apoptosis and stress responses.
- Sphingomyelins comprise a sphingoid base, a fatty acid, and a phosphorylcholine or phosphorylethanolamine head group. These lipids are especially abundant in cell membranes and myelin sheaths, contributing to the stability and insulation of nerve cells.
- Cerebrosides contain a sphingoid base, a fatty acid, and a single sugar residue. Found predominantly in the nervous system, cerebrosides are crucial for maintaining the integrity of neuronal cell membranes.
- Gangliosides are the most complex sphingolipids, featuring multiple sugar residues in addition to the sphingoid base and fatty acid. These lipids are essential in the nervous system for cell-to-cell recognition and signaling.
- Sphingosine-1-phosphate is a phosphorylated sphingoid base that acts as a potent bioactive lipid mediator. S1P is involved in a myriad of cellular processes, including cell migration, proliferation, and immune responses.
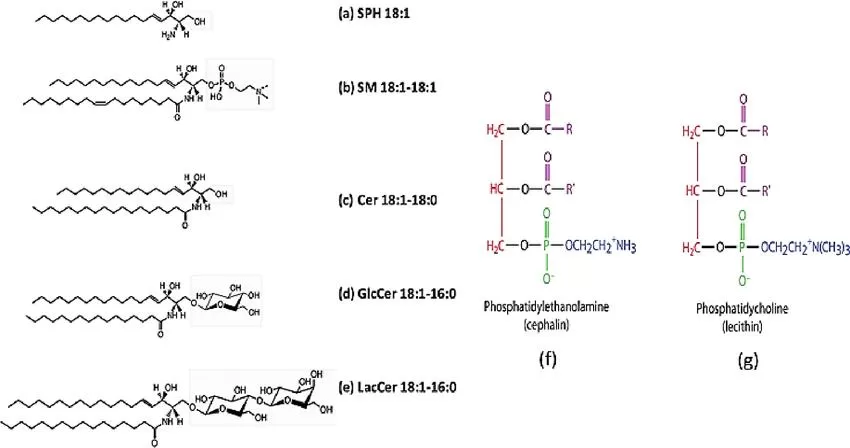
Sphingolipids and phospholipids: The classification of sphingolipids is based on the group attached to the sphingosine (LCB) backbone (Kukwa et al., 2021)
2.Applications of Sphingolipid
Therapeutic Applications
Sphingolipids have emerged as pivotal players in therapeutic applications due to their involvement in cell signaling and regulation. Their roles in apoptosis, cell growth, and differentiation make them attractive targets for drug development. For instance, sphingosine-1-phosphate modulators are being explored for treating autoimmune diseases, given their capacity to influence immune cell trafficking. Additionally, ceramide analogs have shown promise in inducing apoptosis in cancer cells, making them potential candidates for cancer therapy.
In neurodegenerative diseases, sphingolipids are under investigation for their ability to modulate neuronal survival and function. By targeting specific sphingolipid pathways, researchers aim to develop treatments for conditions like Alzheimer's and Parkinson's disease. Moreover, sphingolipids' role in skin barrier function has led to their inclusion in dermatological products aimed at treating conditions such as atopic dermatitis and psoriasis.
Biotechnological Applications
Beyond therapeutics, sphingolipids find significant applications in biotechnology. Their unique properties make them suitable for developing novel biomaterials and drug delivery systems. Liposomes incorporating sphingolipids can enhance the stability and efficacy of encapsulated drugs, offering improved targeting and controlled release. These liposomal formulations are particularly advantageous in cancer therapy, where precise drug delivery is crucial.
In addition, sphingolipids are used in the creation of biosensors and diagnostic tools. Their ability to form stable and functional membrane-like structures enables the development of sensitive and specific detection systems for various biomarkers. This application is vital for early disease diagnosis and monitoring, ensuring timely and accurate medical interventions.
3.Sphingolipid vs. Phospholipid: A Comparative Analysis
Sphingolipids and phospholipids are both indispensable components of cell membranes, each playing crucial roles in cellular functions. However, they exhibit significant differences in their structure and functionality. Let's delve into some of the key distinctions between these two lipid classes:
Backbone Structure
One of the primary differences between sphingolipids and phospholipids lies in their backbone structures. Sphingolipids are characterized by a sphingoid base backbone, which is a long-chain amino alcohol. In contrast, phospholipids have a glycerol backbone. This unique sphingoid base provides sphingolipids with distinct physicochemical properties, differentiating them from the glycerol-based phospholipids.
Fatty Acid Attachment
The attachment of fatty acids also differs between these two lipid classes. In sphingolipids, the fatty acid is linked to the sphingoid base via an amide bond. Conversely, in phospholipids, the fatty acids are attached to the glycerol backbone through ester linkages. This difference in linkage type contributes to the distinct structural and functional properties of sphingolipids and phospholipids.
Polarity
Polarity is another distinguishing factor between sphingolipids and phospholipids. Sphingolipids are generally less polar than phospholipids. This reduced polarity is due to the absence of a charged phosphate group in their structure, which is present in phospholipids. The lower polarity of sphingolipids influences their behavior and interactions within the cell membrane.
Cellular Location
The cellular localization of sphingolipids and phospholipids also differs. Sphingolipids are enriched in specific membrane domains known as lipid rafts. These microdomains play crucial roles in signal transduction and protein trafficking. On the other hand, phospholipids are distributed throughout the entire cell membrane, contributing to membrane fluidity and stability.
Functions
The functions of sphingolipids and phospholipids further highlight their differences. Sphingolipids are involved in a wide range of cellular processes, including apoptosis, cell adhesion, and cell signaling. Phospholipids, however, are primarily responsible for forming the lipid bilayer and compartmentalizing cellular organelles. Despite these functional differences, both lipid classes work synergistically to maintain cellular homeostasis and perform essential roles within cells.
4.Sphingolipid Metabolism
Sphingolipid metabolism encompasses a series of intricate and highly regulated enzymatic reactions that oversee the biosynthesis, degradation, and recycling of sphingolipids. This metabolic pathway is crucial for maintaining cellular homeostasis and is intimately linked to various cellular processes, including cell growth, differentiation, apoptosis, and immune responses.
Sphingolipid Biosynthesis
The biosynthesis of sphingolipids initiates with the condensation of palmitoyl-CoA and serine, forming 3-keto-dihydrosphingosine (3-keto-DHS). This pivotal reaction is catalyzed by the enzyme serine palmitoyltransferase (SPT) and represents the rate-limiting step in sphingolipid biosynthesis. The subsequent steps involve the reduction of 3-keto-DHS to dihydrosphingosine (DHS), followed by acylation to form dihydroceramide, a process catalyzed by dihydroceramide synthase. Finally, dihydroceramide is desaturated by dihydroceramide desaturase to form ceramide, which serves as the backbone for the synthesis of more complex sphingolipids.
Sphingolipid Catabolism
Sphingolipid catabolism primarily occurs in lysosomes and involves the breakdown of sphingolipids into simpler components that can be recycled for further use. This process is mediated by specific enzymes known as sphingolipid hydrolases, including sphingomyelinases, ceramidases, and glycosidases.
Sphingomyelinases
Sphingomyelinases hydrolyze sphingomyelin, a sphingolipid present in the cell membrane, into ceramide and phosphorylcholine. These enzymes play crucial roles in cellular stress responses and the regulation of membrane properties.
Ceramidases
Ceramidases catalyze the hydrolysis of ceramide into sphingosine and a fatty acid. This reaction generates the bioactive lipid sphingosine, which is involved in various cellular processes, including cell apoptosis and immune regulation.
Glycosidases
Glycosidases cleave the sugar residues from complex glycosphingolipids, generating ceramide as a product. This step is critical for the recycling of sphingolipids and the regeneration of ceramide for further use in sphingolipid biosynthesis.
Sphingolipid Recycling and Salvage Pathways
The recycling and salvage pathways of sphingolipids are essential for the efficient reuse of sphingolipid components, thereby conserving cellular resources. These pathways ensure the continuous supply of sphingolipid precursors, such as ceramide and sphingosine, which are vital for the maintenance of cellular sphingolipid levels and function.
During sphingolipid recycling, ceramide can be reacylated to form sphingomyelin or glycosphingolipids, or it can be converted back to sphingosine. The salvage pathways also play a role in the interconversion of sphingolipid metabolites, facilitating their participation in various cellular processes. For instance, sphingosine can be phosphorylated to form sphingosine-1-phosphate , a potent signaling molecule involved in cell migration, proliferation, and immune responses.
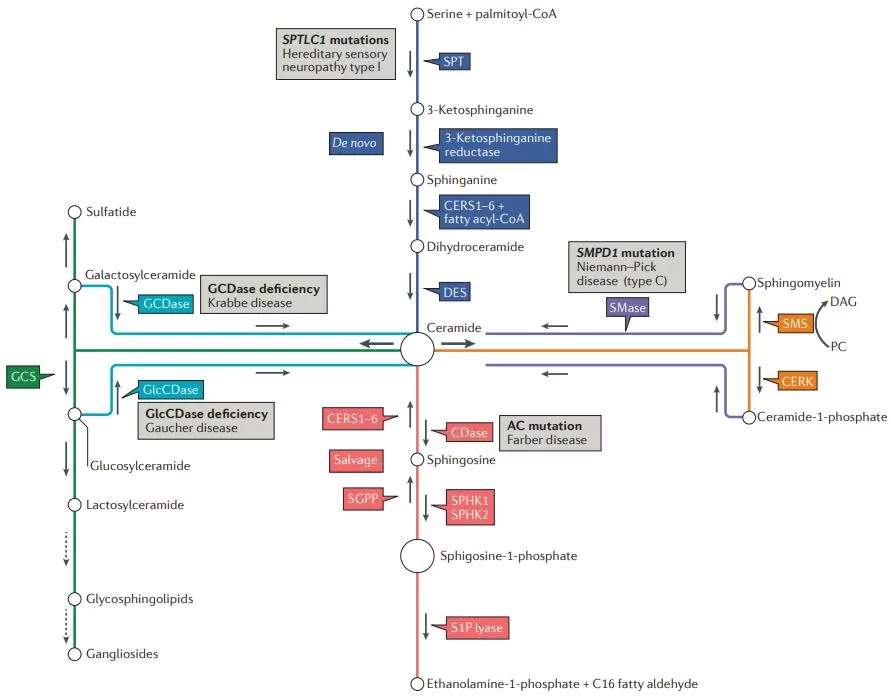
Pathways of sphingolipid metabolism and key enzymes (Ogretmen et al., 2018).
5.Sphingolipid Extraction and Detection
The extraction and detection of sphingolipids are critical steps in studying their structure, function, and role in various biological processes. Efficient extraction methods and precise detection techniques are essential for accurate analysis. This section covers the key methodologies employed in the extraction and detection of sphingolipids.
Sphingolipid Extraction Methods
The extraction of sphingolipids from biological samples involves isolating them from complex mixtures of other lipids, proteins, and cellular components. Commonly used extraction methods include:
Folch Extraction Method
The Folch extraction method is a widely used technique for lipid extraction. It involves the use of a chloroform-methanol mixture to separate lipids from non-lipid components. The process includes homogenizing the sample in a chloroform-methanol solution, followed by the addition of water to induce phase separation. The lower chloroform phase contains the lipids, including sphingolipids, which can be collected for further analysis.
Bligh and Dyer Extraction Method
Similar to the Folch method, the Bligh and Dyer extraction method uses a mixture of chloroform, methanol, and water. The sample is homogenized in the solvent mixture, followed by phase separation. The chloroform phase containing the lipids is collected, dried, and subjected to further purification if necessary.
Solid-Phase Extraction (SPE)
Solid-phase extraction (SPE) is a technique used to purify and concentrate sphingolipids from complex biological mixtures. SPE involves passing the lipid extract through a solid-phase column, which selectively retains the sphingolipids while allowing impurities to be washed away. The sphingolipids are then eluted from the column using an appropriate solvent.
Sphingolipid Detection Methods
After extraction, sphingolipids need to be detected and quantified using reliable analytical techniques. The most commonly employed detection methods are:
Thin-Layer Chromatography (TLC)
Thin-layer chromatography (TLC) is a simple and effective method for separating and identifying sphingolipids. The lipid extract is applied to a TLC plate coated with a stationary phase. The plate is then developed in a solvent system that separates the lipids based on their polarity and affinity for the stationary phase. Sphingolipids can be visualized using specific staining reagents.
High-Performance Liquid Chromatography (HPLC)
High-performance liquid chromatography (HPLC) is a powerful technique for the separation, identification, and quantification of sphingolipids. HPLC involves passing the lipid extract through a chromatographic column under high pressure. The sphingolipids are separated based on their interactions with the column's stationary phase. Detection is achieved using various detectors, such as ultraviolet (UV) or mass spectrometry (MS).
Mass Spectrometry (MS)
Mass spectrometry (MS) is a highly sensitive and specific method for analyzing sphingolipids. In MS, the lipid extract is ionized, and the resulting ions are separated based on their mass-to-charge ratio (m/z). The mass spectra provide detailed information about the sphingolipid species present in the sample. MS can be coupled with chromatography techniques, such as HPLC or gas chromatography (GC), for enhanced separation and analysis.
Nuclear Magnetic Resonance (NMR) Spectroscopy
Nuclear magnetic resonance (NMR) spectroscopy is a non-destructive technique used to elucidate the structure of sphingolipids. NMR provides detailed information about the molecular structure and dynamics of sphingolipids, including the arrangement of functional groups and the conformation of the lipid molecules.
Conclusion: Leveraging Sphingolipid Insights for Advanced Research
The study of sphingolipids offers profound insights into cellular functions and disease mechanisms, highlighting their importance in both fundamental biology and applied sciences. As we continue to unravel the complexities of sphingolipid metabolism and function, innovative technologies and methodologies are essential for advancing our understanding and therapeutic applications. For researchers looking to delve deeper into lipidomics, MetwareBio provides cutting-edge quantitative lipidomics services. With a focus on precision and reliability, MetwareBio is a leading provider in the field, offering comprehensive solutions for lipid analysis. Explore their services at https://www.metwarebio.com/ and learn more about their quantitative lipidomics offerings at Quantitative Lipidomics.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


