What is Central Carbon Metabolism?
Central carbon metabolism lies at the heart of cellular function, orchestrating the conversion of nutrients into energy and building blocks essential for cell growth and survival. In cancer, this metabolic machinery undergoes profound alterations, fueling the uncontrolled proliferation and resilience of malignant cells. Understanding these metabolic rewirings not only sheds light on cancer biology but also opens new avenues for therapeutic innovation.
Central Carbon Metabolism Definition
Central Carbon Metabolism is the fundamental network of biochemical reactions that form the backbone of cellular energy production and biomass synthesis. This intricate process plays a crucial role in maintaining the energy balance and metabolic flexibility of cells, particularly in complex organisms and microbial systems. By efficiently channeling carbon atoms through various metabolic pathways, central carbon metabolism ensures the proper functioning of cellular processes and the generation of essential biomolecules.
At its core, central carbon metabolism integrates multiple key metabolic pathways, including glycolysis, the tricarboxylic acid (TCA) cycle, and the pentose phosphate pathway. These pathways work in synergy to break down carbohydrates, fats, and proteins into simpler molecules, ultimately producing energy in the form of ATP and precursor metabolites needed for biosynthesis and cell growth.
One of the defining characteristics of central carbon metabolism is its ability to adapt to varying environmental and physiological conditions. This adaptability is vital for organisms to thrive under diverse circumstances, such as nutrient availability or stress conditions. The central carbon metabolic network is highly regulated, allowing cells to adjust their metabolic fluxes and prioritize the use of available carbon sources to meet their energetic and biosynthetic needs efficiently.
In industrial applications, a deep understanding of central carbon metabolism is essential for optimizing bioprocesses and enhancing product yields. For example, in the field of biotechnology, manipulating central carbon metabolism can improve the production of biofuels, pharmaceuticals, and other valuable compounds. By engineering specific pathways within the central carbon network, scientists can redirect carbon fluxes towards desired products, increasing efficiency and sustainability.
Enzymes of Central Carbon Metabolism
Central carbon metabolism relies on a diverse array of enzymes that play pivotal roles in catalyzing the various chemical reactions required for efficient carbon utilization. These enzymes are fundamental for the breakdown of complex carbon molecules, the synthesis of crucial biomolecules, and the production of energy-rich compounds. Understanding these enzymes is essential for optimizing metabolic processes in both natural and industrial contexts. Enzymes in central carbon metabolism act as biological catalysts, speeding up reactions that would otherwise proceed too slowly to sustain life. Some of the key enzymes involved include:
Hexokinase
Hexokinase catalyzes the phosphorylation of glucose to glucose-6-phosphate, marking the first step in glucose metabolism. This enzyme's activity is crucial as it facilitates the entry of glucose into the glycolytic pathway, thereby initiating its breakdown for energy production.
Phosphofructokinase
Phosphofructokinase is a rate-limiting enzyme in glycolysis. It catalyzes the conversion of fructose-6-phosphate to fructose-1,6-bisphosphate, effectively controlling the flow of carbon through this vital metabolic pathway. Its regulation is critical for maintaining energy balance within the cell.
Pyruvate Dehydrogenase
Pyruvate dehydrogenase converts pyruvate into acetyl-CoA, a key intermediate that links glycolysis to the tricarboxylic acid (TCA) cycle. This enzyme's function is essential for the further oxidation of glucose-derived carbon and for the production of energy in the form of ATP.
Isocitrate Dehydrogenase
Isocitrate dehydrogenase catalyzes the oxidative decarboxylation of isocitrate to form α-ketoglutarate within the TCA cycle. This reaction is crucial for the continued oxidation of carbon atoms and the production of NADH, which is vital for ATP generation through the electron transport chain.
Malate Dehydrogenase
Malate dehydrogenase converts malate to oxaloacetate in the TCA cycle, regenerating this key intermediate for subsequent rounds of the cycle. This reaction is essential for maintaining the cycle's continuity and for the continuous production of energy.
Each of these enzymes has a specific and vital role within the central carbon metabolic network, contributing to the overall efficiency and adaptability of the metabolic processes. By manipulating these enzymes, particularly in biotechnological and industrial applications, we can enhance the production of valuable metabolites and improve metabolic efficiency.
The Three Central Metabolic Pathways
Central carbon metabolism is structured around three primary metabolic pathways: glycolysis, the pentose phosphate pathway, and the tricarboxylic acid (TCA) cycle. These pathways are interconnected and work together to ensure that cells can efficiently process carbon sources to meet their energy and biosynthetic needs.
Glycolysis
Glycolysis is a universal metabolic pathway occurring in the cytoplasm of all living cells. It involves the step-by-step breakdown of glucose into pyruvate, yielding ATP and NADH. Glycolysis is not only a primary source of energy but also provides essential precursors for various biosynthetic processes.
Pentose Phosphate Pathway
The pentose phosphate pathway runs parallel to glycolysis and serves two main functions: it produces ribose-5-phosphate, a precursor for nucleotide synthesis, and generates NADPH, which is crucial for reductive biosynthesis and combating oxidative stress. This pathway plays a key role in maintaining cellular redox balance and supporting anabolic reactions.
Tricarboxylic Acid (TCA) Cycle
The tricarboxylic acid (TCA) cycle, also known as the citric acid cycle or Krebs cycle, operates in the mitochondria. It completes the oxidation of acetyl-CoA derived from glucose and other carbon sources. The TCA cycle generates NADH and FADH2, which are essential for ATP production via the electron transport chain. Additionally, the TCA cycle provides intermediates for various biosynthetic pathways.
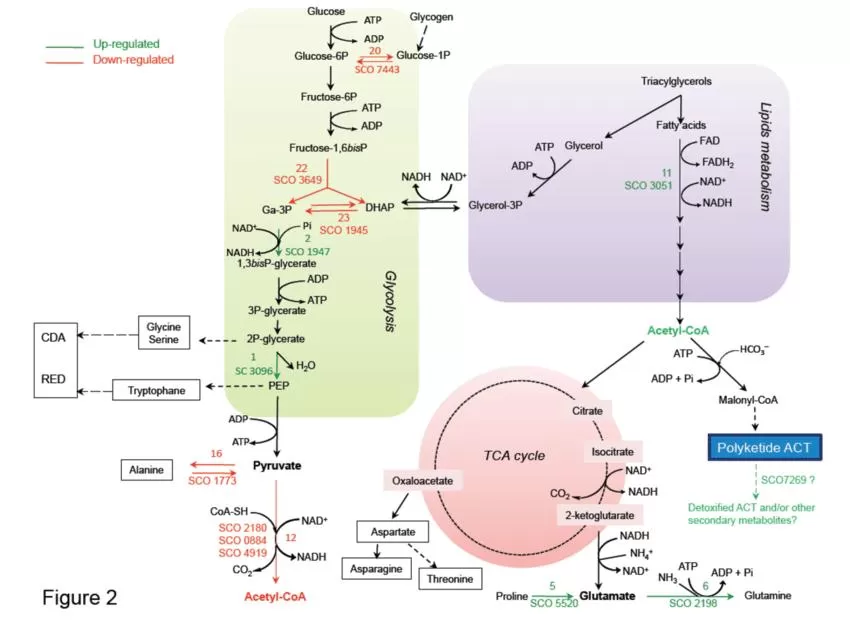
Schematic representation of central metabolic pathways of S.lividans TK24 and its ppk mutant (Le Maréchal et al., 2013)
The Role of Carbon Metabolism
Carbon metabolism is a fundamental aspect of cellular physiology, playing a critical role in maintaining the energy balance, supporting biosynthesis, and regulating cellular functions. This intricate network of metabolic pathways not only ensures the efficient utilization of carbon sources but also integrates various cellular processes to sustain life and drive growth. Understanding the role of carbon metabolism is essential for both biological research and industrial applications, as it underpins the development of advanced technologies and therapeutic strategies.
Energy Production
One of the primary roles of carbon metabolism is the production of energy through the breakdown of carbon-containing molecules such as glucose. This process, which occurs through pathways like glycolysis and the tricarboxylic acid (TCA) cycle, results in the generation of ATP, the universal energy currency of the cell. ATP is crucial for driving a multitude of energy-dependent processes, including cellular growth, movement, and signaling. The efficient conversion of glucose into ATP provides the necessary energy to sustain various cellular activities and maintain homeostasis.
In addition to ATP production, carbon metabolism supports the storage of energy in the form of glycogen and lipids. These energy reserves are mobilized during periods of energy demand, ensuring a continuous supply of ATP even under conditions of nutrient scarcity. This dynamic energy balance is vital for the survival and functionality of cells, allowing them to adapt to changing environmental conditions.
Biosynthesis
Carbon metabolism also plays a key role in biosynthesis, providing the precursor molecules needed for the construction of essential cellular components. Intermediates from central metabolic pathways such as glycolysis and the TCA cycle are utilized to synthesize a wide array of biomolecules, including amino acids, nucleotides, and lipids. These building blocks are crucial for protein synthesis, DNA replication, and membrane formation, supporting the growth, repair, and maintenance of cells.
For example, the intermediate pyruvate produced during glycolysis can be converted into acetyl-CoA, a key substrate for the synthesis of fatty acids and cholesterol. Similarly, intermediates from the TCA cycle, such as oxaloacetate and α-ketoglutarate, are precursors for the synthesis of non-essential amino acids, which are vital for protein production and other cellular functions.
Redox Balance
Maintaining the cellular redox balance is another critical function of carbon metabolism. The pathways involved in carbon metabolism generate reducing equivalents in the form of NADH and NADPH, which serve as electron carriers in numerous biochemical reactions. NADH is primarily involved in oxidative phosphorylation, where it donates electrons to the electron transport chain to produce ATP. This process is essential for the efficient generation of energy and the maintenance of a healthy cellular environment.
On the other hand, NADPH is crucial for anabolic processes and antioxidant defense. It provides the reducing power required for the synthesis of fatty acids, nucleotides, and other biomolecules, as well as for the detoxification of reactive oxygen species through the regeneration of reduced glutathione. By balancing the production and utilization of NADH and NADPH, carbon metabolism helps to maintain cellular redox homeostasis and protect against oxidative damage.
Regulation of Cell Signaling
Metabolites generated during central carbon metabolism also play important roles as signaling molecules that regulate various cellular processes. These metabolites can function as allosteric regulators, influencing the activity of key enzymes and modulating gene expression in response to the metabolic state of the cell. For instance, intermediates such as fructose-2,6-bisphosphate can activate or inhibit enzymes in glycolysis and gluconeogenesis, thereby regulating the flux of carbon through these pathways based on the cellular energy status.
Moreover, changes in the levels of certain metabolites can trigger signaling cascades that influence cell growth, differentiation, and apoptosis. This intricate regulatory network ensures that cells can respond adaptively to metabolic and environmental cues, coordinating their metabolic activities with broader physiological functions.
Regulation Mechanisms of Central Carbon Metabolism
Central carbon metabolism is a highly regulated network, essential for maintaining cellular energy balance, biosynthesis, and overall metabolic homeostasis. Various mechanisms ensure that metabolic pathways respond efficiently to changes in environmental conditions and cellular demands. These regulatory mechanisms include allosteric regulation, feedback inhibition, hormonal control, transcriptional regulation, post-translational modifications, and substrate availability. Each plays a critical role in fine-tuning the activity of enzymes and the flow of metabolites through the central carbon metabolic pathways.
Allosteric Regulation
Allosteric regulation involves the binding of effector molecules to specific sites on enzymes, distinct from the active site. This binding can either activate or inhibit enzyme activity, depending on the nature of the effector. In central carbon metabolism, key enzymes are subject to allosteric regulation to modulate the rate of metabolic pathways in response to cellular energy levels.
For instance, in glycolysis, the enzyme phosphofructokinase-1 (PFK-1) is allosterically inhibited by high levels of ATP, which indicates a sufficient energy supply and slows down the glycolytic flux. Conversely, AMP, which signals low energy status, acts as an allosteric activator of PFK-1, promoting glycolysis to generate more ATP. This ensures that glucose is efficiently utilized based on the energy needs of the cell.
Feedback Inhibition
Feedback inhibition is a critical regulatory mechanism where the end product of a metabolic pathway inhibits an enzyme that acts earlier in the pathway. This negative feedback loop helps to maintain metabolic balance by preventing the over-accumulation of end products.
In the case of glycolysis, ATP acts as a feedback inhibitor of PFK-1, thus regulating its own production by limiting the entry of glucose into the pathway when energy levels are high. Similarly, citrate, an intermediate of the TCA cycle, can inhibit PFK-1, linking the rate of glycolysis to the cell's overall energy status and metabolic activity.
Hormonal Control
Hormones play a pivotal role in regulating central carbon metabolism, particularly in response to changes in blood glucose levels. Hormones such as insulin and glucagon orchestrate the metabolic response to feeding and fasting, respectively.
Insulin, released in response to high blood glucose, promotes glucose uptake and utilization by stimulating glycolysis and glycogenesis. It activates key enzymes such as hexokinase and phosphofructokinase, facilitating glucose conversion into energy and storage forms. In contrast, glucagon is released during low blood glucose levels and stimulates glycogenolysis and gluconeogenesis, increasing glucose availability for energy production. These hormonal signals ensure that glucose levels are tightly regulated, maintaining metabolic homeostasis.
Regulation of Transcription
Transcriptional regulation involves the control of gene expression, which dictates the levels of enzymes involved in central carbon metabolism. Cells adapt to changes in nutrient availability and metabolic demands by adjusting the transcription of genes encoding metabolic enzymes.
Signaling pathways and transcription factors respond to metabolic cues, modulating the expression of genes involved in glycolysis, the TCA cycle, and other pathways. For example, the transcription factor HIF-1 (hypoxia-inducible factor 1) upregulates the expression of glycolytic enzymes under low oxygen conditions, enhancing glycolysis and promoting survival under hypoxic stress.
Post-translational Modifications
Post-translational modifications (PTMs) such as phosphorylation, acetylation, and methylation can significantly influence enzyme activity. These reversible modifications allow for rapid and dynamic regulation of metabolic pathways.
For example, phosphorylation of enzymes in glycolysis and gluconeogenesis can activate or deactivate these enzymes in response to changes in cellular energy status. The enzyme pyruvate kinase, which catalyzes the final step in glycolysis, is regulated by phosphorylation, where phosphorylation by protein kinase A (PKA) inhibits its activity under low glucose conditions, redirecting pyruvate toward gluconeogenesis.
Substrate Availability
Substrate availability is a crucial factor influencing central carbon metabolism. The concentration of key metabolites directly affects the rate of metabolic pathways. For example, the availability of glucose determines the rate of glycolysis, while the supply of acetyl-CoA influences the activity of the TCA cycle.
When glucose levels are low, cells can shift to alternative carbon sources such as fatty acids and amino acids, which enter central carbon metabolism at different points. This flexibility allows cells to maintain energy production and biosynthesis under varying nutrient conditions, ensuring metabolic resilience and adaptability.
Methods of Central Carbon Metabolism Analysis
Central carbon metabolism is a complex network of biochemical pathways essential for cellular energy production, biosynthesis, and overall metabolic homeostasis. To study these pathways and understand their role in health and disease, researchers employ a variety of advanced analytical techniques. Here, we explore some of the key methods used for analyzing central carbon metabolism.
Stable Isotope Tracing
Stable isotope tracing is a powerful technique for investigating metabolic pathways and fluxes within central carbon metabolism. This method involves introducing stable, isotopically labeled substrates into a biological system and tracking their incorporation into metabolites over time. The labeled isotopes, such as ^13C or ^15N, act as tracers, providing valuable insights into the fate of carbon atoms and the activity of specific metabolic pathways.
The process typically involves culturing cells or organisms in the presence of isotopically labeled compounds, like ^13C-labeled glucose or ^15N-labeled amino acids. As these substrates are metabolized, the isotopic label gets incorporated into metabolic intermediates and end products. By analyzing the isotopic enrichment patterns of metabolites using techniques such as mass spectrometry or nuclear magnetic resonance (NMR) spectroscopy, researchers can trace the metabolic pathways, reveal information about pathway activities, reaction rates, and carbon fluxes.
Metabolomics
Metabolomics is the comprehensive analysis of metabolites within a biological system. This approach involves the systematic identification and quantification of small molecules, including intermediates, end products, and signaling molecules involved in central carbon metabolism. Metabolomics provides a detailed snapshot of the metabolic state and can reveal changes in metabolite concentrations or profiles in response to different cellular conditions or genetic perturbations.
Using techniques such as gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS), or NMR spectroscopy, researchers can profile a wide array of metabolites. This comprehensive data helps in identifying key metabolic changes associated with diseases, understanding metabolic pathway interactions, and discovering potential biomarkers for diagnostic or therapeutic purposes.
Fluxomics
Fluxomics focuses on the quantification of metabolic fluxes, which represent the rates of biochemical reactions and pathway activities within central carbon metabolism. Fluxomics provides a dynamic view of metabolic networks by measuring the flow of metabolites through different pathways and identifying bottlenecks or rate-limiting steps.
One common approach to fluxomics involves the use of stable isotopes as tracers. By measuring the incorporation of these isotopes into metabolites over time, researchers can determine metabolic fluxes. Mathematical models, such as flux balance analysis (FBA) or kinetic models, are then applied to interpret the isotopic labeling data and estimate metabolic fluxes. These models help in understanding the metabolic capacity of cells and predicting how changes in environmental or genetic conditions can impact metabolic flows.
Select Services
Several specialized services are available for researchers seeking to analyze central carbon metabolism. These services offer advanced analytical techniques and expertise to support various aspects of metabolic research. Key services include:
- Stable Isotope Tracing Services: Providers offer tailored isotope tracing studies, including experimental design, isotopic labeling, sample preparation, and data analysis to track metabolic pathways and fluxes.
- Metabolomics Services: Comprehensive metabolite profiling services, including sample preparation, high-throughput analysis, and data interpretation, help uncover metabolic alterations and potential biomarkers.
- Fluxomics Services: Specialized services include metabolic flux analysis, isotopic labeling studies, and computational modeling to quantify and analyze metabolic fluxes within biological systems.
- Custom Metabolic Studies: Providers offer bespoke studies tailored to specific research needs, including custom assay development, targeted metabolite analysis, and integrated multi-omics approaches to explore central carbon metabolism.
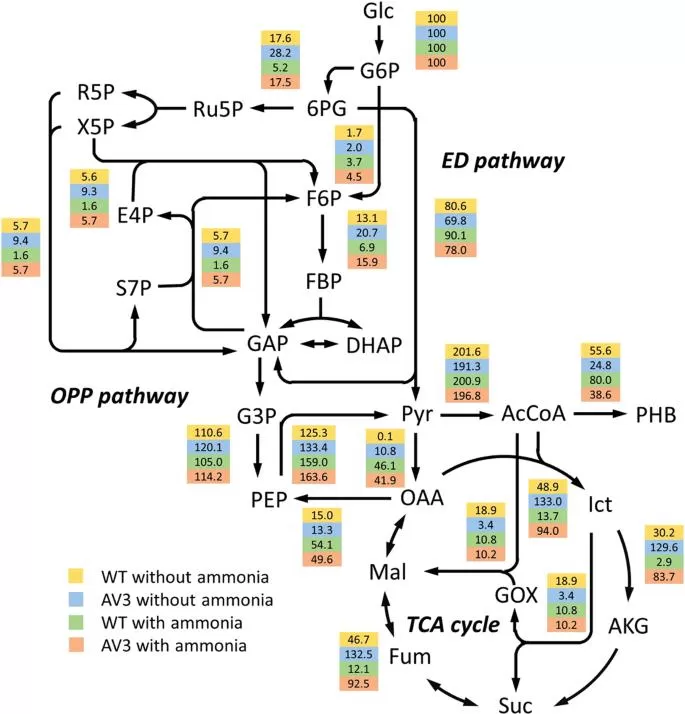
Metabolic flux maps of A. vinelandii wt and AV3 with and without ammonium. (Wu et al., 2019)
Application of Central Carbon Metabolism Analysis in Cancer
Cancer cells exhibit significant metabolic alterations compared to normal cells, with central carbon metabolism playing a pivotal role in supporting their rapid proliferation and survival. Analyzing central carbon metabolism in the context of cancer provides critical insights that can inform novel therapeutic strategies and improve cancer treatment outcomes.
Biomarker Discovery
Metabolic profiling of cancer cells enables the identification of unique metabolic signatures that differentiate them from normal cells. These metabolic signatures serve as potential biomarkers for cancer detection, patient stratification based on metabolic phenotypes, and monitoring treatment response.
Target Identification
Central carbon metabolism analysis helps pinpoint metabolic enzymes or pathways essential for cancer cell survival and proliferation. Targeting these metabolic vulnerabilities can lead to the development of innovative anticancer therapies that selectively inhibit cancer cell metabolism.
Therapeutic Monitoring
Monitoring changes in central carbon metabolism during cancer treatment provides valuable insights into treatment efficacy and tumor response. Metabolic imaging techniques, such as positron emission tomography (PET) using glucose analogs, allow for non-invasive assessment of tumor metabolic activity and response to therapeutic interventions.
Metabolic Reprogramming
Understanding the metabolic rewiring in cancer cells informs the development of metabolic inhibitors or modulators that disrupt altered metabolic pathways. These therapeutic approaches aim to selectively target cancer metabolism while minimizing adverse effects on normal cells.
Personalized Medicine
Central carbon metabolism analysis contributes to personalized medicine by identifying metabolic alterations specific to individual patients or cancer subtypes. This personalized approach enables tailored therapeutic strategies that optimize treatment outcomes and patient survival rates.
Advancing Cancer Treatment through Metabolic Insights
By unraveling the complexities of central carbon metabolism in cancer, researchers are pioneering targeted therapies that promise improved outcomes for patients worldwide. Innovators like MetwareBio lead the charge in metabolomics, lipidomics, and proteomics providing cutting-edge solutions to decode cancer's metabolic signatures and transform treatment strategies. Explore the future of cancer care at MetwareBio, where precision meets innovation.
References
-
Le Maréchal, Pierre, et al. "Comparative proteomic analysis of Streptomyces lividans Wild-Type and ppk mutant strains reveals the importance of storage lipids for antibiotic biosynthesis." Applied and environmental microbiology 79.19 (2013): 5907-5917.
-
Wu, Chao, et al. "Fluxomic analysis reveals central carbon metabolism adaptation for diazotroph azotobacter vinelandii ammonium excretion." Scientific reports 9.1 (2019): 13209.


