Acyl-CoA: Biological Function and Analytical Methods
Acyl-CoA plays a pivotal role in cellular metabolism, acting as a critical intermediary in various biochemical processes. From energy production to lipid biosynthesis, this versatile molecule is central to numerous metabolic pathways. Understanding Acyl-CoA and its functions is essential for advancing research in metabolic profiling, disease biomarker discovery, pharmacological studies, and more. In this comprehensive guide, we delve into the intricacies of Acyl-CoA metabolism, exploring its significance and the analytical methods used to study it.
What is Acyl-CoA?
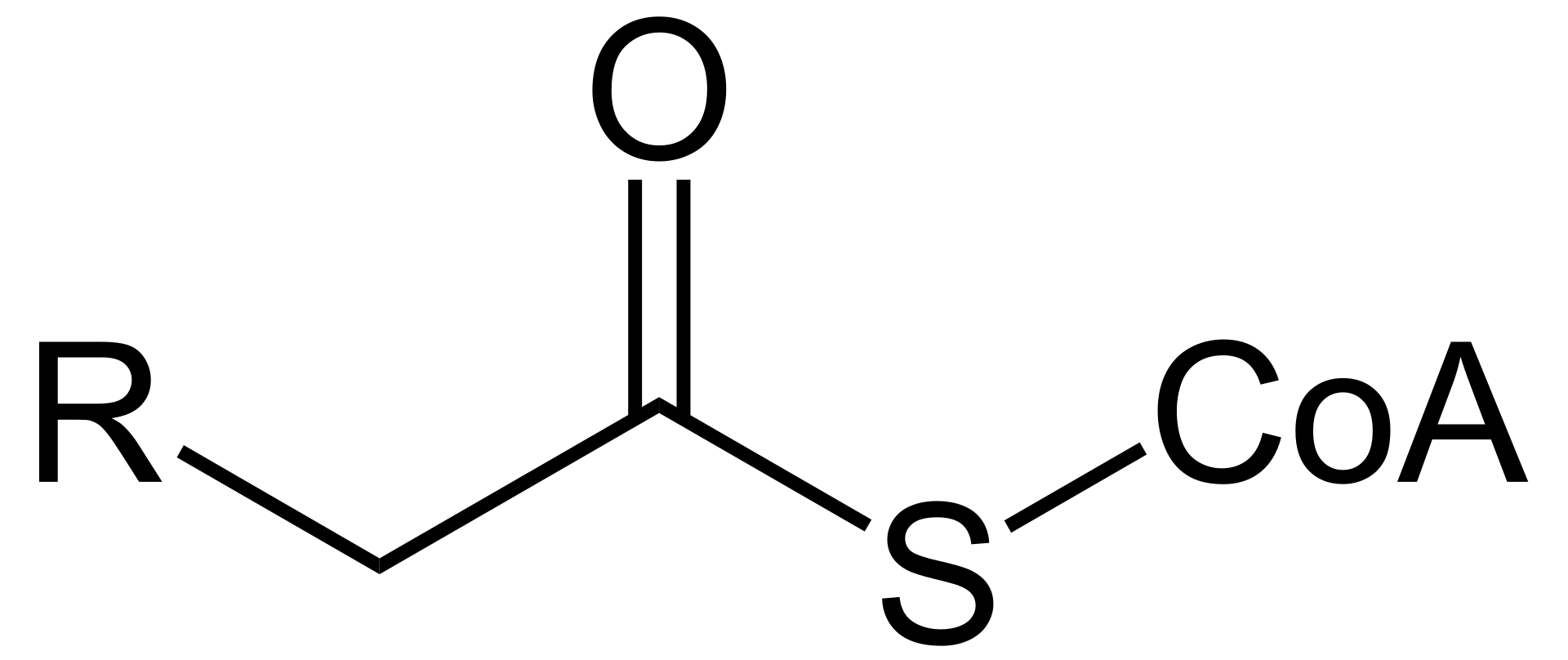
Acyl-CoA, a vital molecule in the biochemical world, plays a significant role in various metabolic pathways. But what exactly is Acyl-CoA? Let’s break it down. The term "Acyl-CoA" refers to a group of coenzymes involved in the metabolism of fatty acids and other acyl compounds. These coenzymes are essentially the activated forms of fatty acids, which means they are ready to participate in various biochemical reactions.
At its core, Acyl-CoA is formed when a fatty acid is conjugated to coenzyme A (CoA), a process catalyzed by the enzyme Acyl-CoA synthetase. This combination forms a thioester bond, linking the acyl group to CoA. This transformation is crucial because it enables the fatty acid to enter metabolic pathways, such as beta-oxidation, which is pivotal for energy production.
Understanding Acyl-CoA is fundamental for those in the biochemical and pharmaceutical industries, as it plays a key role in lipid metabolism, signaling, and even gene expression. The versatility and importance of Acyl-CoA make it a cornerstone in the study of metabolism and bioenergetics.
Imagine Acyl-CoA as a sort of biochemical currency, facilitating the exchange and transformation of energy within cells. Its presence is critical for the synthesis of complex lipids, degradation of fatty acids, and regulation of metabolic pathways. In a way, Acyl-CoA acts as a molecular switch, turning on and off various metabolic processes as needed.
For businesses and researchers in the biochemical and pharmaceutical fields, comprehending the intricacies of Acyl-CoA can unlock new avenues for innovation and therapeutic development. As we delve deeper into the specifics of Acyl-CoA, its functions, metabolism, and the analytical methods used to study it, we will uncover the profound impact this molecule has on our understanding of biochemistry and human health.
Stay tuned as we explore the fascinating world of Acyl-CoA, a molecule that is as intriguing as it is essential to life.
Functions of Acetyl-CoA
Acetyl-CoA is an essential intermediate in cellular metabolism, acting as a pivotal molecule in various metabolic processes. Its significance extends far beyond being merely a product of catabolic reactions; Acetyl-CoA is also a starting metabolite for lipid biosynthesis, thereby supporting cell growth and proliferation.
One of the most remarkable roles of Acetyl-CoA is in protein acetylation reactions. The cellular levels of Acetyl-CoA dynamically correlate with the acetylation levels of histones and transcription factors, playing a significant role in epigenetic regulation. This correlation highlights its crucial role in gene expression and other cellular processes, making Acetyl-CoA indispensable for proper cellular function.
Acetyl-CoA is predominantly distributed in the mitochondria, nucleus, and cytoplasm of cells. Mitochondrial Acetyl-CoA is often regarded as a relatively independent component, while nuclear and cytoplasmic Acetyl-CoA are considered a collective entity, referred to as the nucleocytoplasmic fraction. This distribution is essential for understanding how Acetyl-CoA impacts various cellular processes differently based on its location.
Research has shown that changes in mitochondrial or nucleocytoplasmic Acetyl-CoA levels can significantly affect the acetylation levels of histones. Acetyl-CoA serves as an essential cofactor for histone acetyltransferases (HATs), enzymes that acetylate histones, thereby influencing chromatin structure and gene expression. The ratio of Acetyl-CoA to CoA is crucial in regulating the enzymatic activity and specificity of HATs, underscoring the importance of Acetyl-CoA in epigenetic regulation.
For industries involved in biochemistry and pharmaceuticals, understanding the functions of Acetyl-CoA can provide insights into developing novel therapeutic strategies. By manipulating Acetyl-CoA levels or its metabolic pathways, it may be possible to influence cellular processes such as energy production, lipid biosynthesis, and gene expression, offering potential avenues for innovative treatments.
Mitochondrial Acyl-CoA Metabolism
Mitochondrial Acyl-CoA metabolism is fundamental for energy production within cells. This complex process involves the generation of Acetyl-CoA from pyruvate through the action of pyruvate dehydrogenase within the mitochondria. Once formed, Acetyl-CoA serves as a central hub for various metabolic pathways, including the tricarboxylic acid (TCA) cycle, which is crucial for generating reducing equivalents necessary for ATP production.
In addition to its role in the TCA cycle, Acetyl-CoA is also a key player in the biosynthesis of fatty acids and other essential molecules. The process of fatty acid synthesis begins with Acetyl-CoA as a substrate, where the enzyme Acetyl-CoA carboxylase converts it into malonyl-CoA. This molecule is further processed to produce long-chain fatty acids, which are vital components of cell membranes and energy storage molecules.
Acyl-CoA molecules derived from fatty acids also participate in mitochondrial beta-oxidation, a metabolic process that breaks down fatty acids to generate energy. Enzymes such as acyl-CoA dehydrogenase facilitate the oxidation of Acyl-CoA molecules of different chain lengths, enabling the stepwise degradation of fatty acids into Acetyl-CoA units, which then enter the TCA cycle to produce ATP.
The significance of mitochondrial Acyl-CoA metabolism extends to its involvement in various cellular functions and energy homeostasis. However, inherited deficiencies in enzymes related to this metabolic pathway can lead to fatty acid oxidation disorders. These disorders are characterized by impaired fatty acid breakdown, leading to the accumulation of toxic metabolites and a range of clinical symptoms. Understanding these metabolic processes and their associated enzymes is crucial for diagnosing and treating metabolic disorders effectively.
For researchers and professionals in the biochemical and pharmaceutical industries, a thorough grasp of mitochondrial Acyl-CoA metabolism opens the door to potential therapeutic innovations. By targeting specific enzymes or pathways, it may be possible to develop treatments for metabolic disorders or enhance energy production in cells, offering new possibilities for managing various health conditions.
Cytoplasmic Acyl-CoA Metabolism
In the cytoplasm, Acyl-CoA metabolism is predominantly linked to the synthesis and metabolism of fatty acids. Fatty acids can be derived from dietary sources or synthesized de novo within the body. Regardless of their origin, fatty acids must be activated before participating in various metabolic processes.
The activation of fatty acids is facilitated by enzymes known as fatty acyl-CoA synthetases, or fatty acid thiokinases. These enzymes catalyze the ATP-dependent formation of fatty acyl-CoA molecules by conjugating a fatty acid with coenzyme A (CoA). The resulting fatty acyl-CoA molecules are then primed for subsequent metabolic reactions.
Once activated, fatty acyl-CoA molecules have several potential metabolic pathways depending on the cell's energy demands and metabolic state. They can be directed towards mitochondrial beta-oxidation, where they are broken down to produce Acetyl-CoA and generate energy. Alternatively, these molecules can be utilized for the synthesis of complex lipids such as phospholipids, triglycerides, and cholesterol esters, which are essential components of cell membranes and lipid droplets.
In addition, fatty acids can be elongated or desaturated to produce a variety of fatty acid species with specific functions. These reactions occur in the endoplasmic reticulum and involve enzymes known as fatty acid elongases and desaturases. Acyl-CoA molecules serve as substrates for these enzymatic reactions, contributing to the production of fatty acids with varying chain lengths and degrees of unsaturation.
The regulation of cytoplasmic Acyl-CoA metabolism is meticulously controlled to maintain cellular homeostasis. Enzymes involved in fatty acid synthesis, such as acetyl-CoA carboxylase and fatty acid synthase, are regulated by hormonal and nutritional signals. The availability of substrates like Acetyl-CoA and malonyl-CoA, along with the cell's energy status, influences the activity of these enzymes and the overall balance of fatty acid synthesis and metabolism.
Understanding the complexities of cytoplasmic Acyl-CoA metabolism is crucial for those in the biochemical and pharmaceutical sectors. Insights into these pathways can lead to the development of targeted therapies for metabolic disorders and innovative strategies for manipulating lipid metabolism to improve health outcomes.
Distinction between Acyl-CoA and Acetyl-CoA
Acyl-CoA and Acetyl-CoA, although structurally similar, serve different roles within cellular metabolism due to their distinct acyl groups. Acyl-CoA represents a broad category of molecules, each consisting of a coenzyme A (CoA) moiety linked to an acyl group. This acyl group can vary widely, being derived from fatty acids with different chain lengths and degrees of saturation. This variability allows acyl-CoA to participate in a diverse array of metabolic pathways, influencing numerous cellular functions and processes.
In contrast, Acetyl-CoA is a specific type of acyl-CoA where the acyl group is derived from acetic acid, a two-carbon molecule. Acetyl-CoA plays a crucial role as an intermediate in energy metabolism, particularly within the tricarboxylic acid (TCA) cycle, where it contributes to the production of ATP, the energy currency of the cell. Additionally, Acetyl-CoA is a key substrate in the biosynthesis of fatty acids and cholesterol, and it also serves as an important regulator in the process of protein acetylation, impacting gene expression and cellular function.
Understanding the distinction between acyl-CoA and acetyl-CoA is essential for comprehending their respective roles in metabolism. While acetyl-CoA is critical for energy production and biosynthetic pathways, acyl-CoA encompasses a wider range of molecules that are involved in various metabolic functions, from fatty acid oxidation to complex lipid synthesis. This distinction highlights the versatility and specificity of CoA derivatives in maintaining cellular homeostasis and metabolic regulation.
Application of Acyl-CoA Analysis in Research
Acyl-CoA analysis is a powerful tool in scientific research, offering profound insights into cellular metabolism and its dysregulation in various diseases. By accurately measuring and characterizing acyl-CoA species, researchers can better understand metabolic pathways, identify biomarkers, and evaluate therapeutic interventions. Below are several key applications of Acyl-CoA analysis in research:
1. Metabolic Profiling and Pathway Analysis
Acyl-CoA analysis facilitates comprehensive profiling of acyl-CoA species in different cellular compartments and tissues. Using mass spectrometry-based approaches, researchers can identify and quantify specific acyl-CoA molecules, offering a snapshot of the metabolic state of cells or organisms. This profiling enables the elucidation of metabolic pathways and the identification of key regulatory steps, deepening our understanding of cellular metabolism.
2. Disease Biomarker Discovery
Altered acyl-CoA metabolism is linked to numerous metabolic disorders, including fatty acid oxidation disorders, obesity, diabetes, and cancer. Analyzing acyl-CoA species can help identify disease-specific metabolic signatures and potential biomarkers. By comparing acyl-CoA profiles between healthy and diseased individuals, researchers can discover novel biomarkers that reflect the dysregulation of specific metabolic pathways. These biomarkers are promising for early disease detection, monitoring disease progression, and assessing treatment responses.
3. Pharmacological Studies
Acyl-CoA analysis is invaluable for evaluating the efficacy and safety of drugs targeting metabolic pathways. Researchers can assess the impact of pharmacological interventions on acyl-CoA profiles to determine the drug's effects on specific metabolic pathways and potential off-target effects. This information aids in optimizing drug dosing, developing personalized therapies, and identifying potential drug-drug interactions.
4. Mechanistic Studies
Studying acyl-CoA metabolism provides insights into the underlying mechanisms of metabolic diseases and disorders. By manipulating specific enzymes or pathways involved in acyl-CoA metabolism, researchers can investigate the consequences of altered acyl-CoA levels or distribution. These mechanistic studies help unravel the molecular basis of metabolic disorders, contributing to the development of targeted therapeutic strategies.
5. Nutritional and Dietary Research
Acyl-CoA analysis plays a critical role in understanding the effects of nutrition and dietary interventions on cellular metabolism. Researchers can assess changes in acyl-CoA profiles in response to different dietary components, such as fats, carbohydrates, or specific nutrients. This analysis helps elucidate the impact of dietary factors on metabolic pathways and contributes to the development of personalized dietary recommendations for health promotion and disease prevention.
Analytical Methods for Studying Acyl-CoA
Accurate detection and quantification of Acyl-CoA and its metabolic pathways require reliable analytical methods. Among these, mass spectrometry (MS) has emerged as a highly sensitive and specific tool for Acyl-CoA analysis. Various MS-based approaches have been developed to investigate Acyl-CoA, including:
Importance of Accurate Measurement
Accurate measurement of Acyl-CoA levels is crucial for understanding cellular metabolism and its alterations in various diseases. Precise quantification helps in profiling metabolic pathways, identifying biomarkers, and assessing the impact of therapeutic interventions. Without reliable measurements, the interpretation of metabolic data can be misleading, affecting the conclusions drawn from research studies.
Chromatography Methods
Chromatography is a key analytical technique used in the separation and analysis of Acyl-CoA species. Two main types of chromatography methods used for Acyl-CoA analysis are gas chromatography (GC) and liquid chromatography (LC).
Gas Chromatography
Gas chromatography (GC) is a powerful technique for separating volatile Acyl-CoA derivatives. By converting Acyl-CoA molecules into their volatile forms, GC allows for the efficient separation and analysis of different species. GC is particularly useful in applications where high-resolution separation of Acyl-CoA derivatives is required.
Liquid Chromatography
Liquid chromatography (LC) is more versatile and widely used in metabolomics studies. LC allows for the separation of Acyl-CoA species in their native forms without the need for derivatization. When combined with mass spectrometry (LC-MS), this technique enables the identification and quantification of different Acyl-CoA species present in complex biological samples, making it suitable for comprehensive profiling of Acyl-CoA metabolism.
Mass Spectrometry
Mass spectrometry (MS) is a cornerstone technique in the analysis of Acyl-CoA species due to its sensitivity and specificity. Various MS-based approaches enhance the study of Acyl-CoA metabolism:
Tandem Mass Spectrometry (MS/MS)
Tandem Mass Spectrometry (MS/MS) involves the sequential use of two mass analyzers to provide structural information about the analyzed molecules. By subjecting Acyl-CoA species to fragmentation, MS/MS aids in the identification and differentiation of various Acyl-CoA molecules. This technique is particularly useful for studying Acyl-CoA isomers or distinguishing between different Acyl-CoA species with similar masses.
Isotope Dilution Mass Spectrometry (IDMS)
For precise measurement of Acyl-CoA species, isotope dilution mass spectrometry (IDMS) uses internal standards tagged with stable isotopes. IDMS enables accurate estimation of Acyl-CoA concentrations by comparing the signals of endogenous Acyl-CoA species with the tagged standards. This technique is excellent for quantitative analysis and evaluating Acyl-CoA levels in biological samples due to its high accuracy and dependability.
High-Resolution Mass Spectrometry (HRMS)
High-Resolution Mass Spectrometry (HRMS) offers enhanced mass accuracy and resolving power, enabling precise determination and characterization of Acyl-CoA species. It is particularly useful for identifying and structurally analyzing Acyl-CoA molecules with complex or modified structures. HRMS is valuable for in-depth investigations of Acyl-CoA metabolism and detecting subtle metabolic alterations.
Leading Proteomics Solutions with MetwareBio
The study of Acyl-CoA and its various roles in cellular metabolism is vital for advancing our understanding of biochemical processes and disease mechanisms. Accurate measurement and analysis of Acyl-CoA species are essential for uncovering metabolic pathways, identifying potential biomarkers, and developing targeted therapeutic strategies. Whether you're engaged in metabolic profiling, disease biomarker discovery, pharmacological studies, or nutritional research, the insights gained from Acyl-CoA analysis are invaluable.
For cutting-edge proteomics solutions, MetwareBio offers unparalleled expertise and advanced technologies. With a global reputation for excellence, MetwareBio is your trusted partner in proteomics research. Visit https://www.metwarebio.com/ to learn more about their comprehensive range of services and how they can support your research endeavors.


