What Are Nucleotides? Discover the Molecules Behind DNA, Immunity, and Vitality
When we think about what makes life possible, it's easy to picture cells, organs, or even DNA. But hidden beneath these familiar biological structures lies a far more fundamental layer: nucleotides. These small but mighty molecules form the essence of life’s code—and yet, their influence stretches far beyond what most people realize. They don’t just store genetic information; they energize our bodies, keep our cells communicating, and quietly shape our health in ways we’re only beginning to understand. From the moment we wake up to the cellular decisions unfolding in our brains and immune systems, nucleotides are working behind the scenes. So, what exactly are these molecular powerhouses? And why should we care about them? Let’s dive in and uncover the incredible world of nucleotides—where life’s smallest components carry the weight of our very existence.
From Discovery to Molecular Marvel: The Story and Structure of Nucleotides
The journey of discovering nucleotides began with the pioneering work of Friedrich Miescher in 1869, who extracted a novel phosphorus-containing substance from pus cells, which he termed "nuclein." This substance was later identified as DNA. In the early 20th century, biochemist Phoebus Levene made significant strides in understanding nucleotide composition, identifying the three fundamental components: nitrogenous base, sugar, and phosphate. He also proposed the "tetranucleotide hypothesis," which, although incorrect, paved the way for further research. It wasn’t until 1953 that Watson and Crick, building on Rosalind Franklin's X-ray diffraction images, unveiled the double helix structure of DNA, revolutionizing molecular biology.
Structurally, nucleotides are composed of three components: a nitrogenous base (either a purine such as adenine and guanine, or a pyrimidine such as cytosine, thymine, or uracil), a pentose sugar (ribose in RNA or deoxyribose in DNA), and one to three phosphate groups. The phosphate groups are linked to the 5’ carbon of the sugar, while the base is attached to the 1’ carbon. These monomers polymerize through phosphodiester bonds between the 3’ hydroxyl group of one sugar and the 5’ phosphate of the next, forming the sugar-phosphate backbone of nucleic acids. The specific sequence of the nitrogenous bases encodes genetic information, while their ability to hydrogen bond underlies the formation of DNA's iconic double-helix structure.
 and five nitrogenous bases (B)_1745283536_WNo_941d426.webp)
The structure of nucleotides (A) and five nitrogenous bases (B)
The Blueprint Factory: Biosynthesis of Nucleotides
The production of nucleotides within cells is a meticulously orchestrated process that ensures a steady supply of these essential molecules for growth, repair, and survival. Cells utilize two primary strategies—de novo synthesis and salvage pathways—to generate purine and pyrimidine nucleotides, each involving distinct enzymes, substrates, and regulatory checkpoints.
Purine Biosynthesis: Constructing Complexity Atom by Atom
For purines, the de novo pathway is a highly coordinated and energy-intensive process that begins with the activation of ribose-5-phosphate, a sugar derived from the pentose phosphate pathway. This molecule is converted into phosphoribosyl pyrophosphate (PRPP) by PRPP synthetase (encoded by the PRPS1 gene). PRPP then serves as the scaffold for building the purine ring directly onto the ribose backbone. A series of enzymatic reactions sequentially adds atoms from sources including glycine, glutamine, aspartate, carbon dioxide, and N10-formyltetrahydrofolate. Notable enzymes involved include GART, PAICS, and ATIC. The final product of this synthetic sequence is inosine monophosphate (IMP), which acts as the branching point for the synthesis of adenosine monophosphate (AMP) and guanosine monophosphate (GMP). This process is tightly regulated by feedback inhibition from the end products to avoid overproduction.
Pyrimidine Biosynthesis: Synthesizing the Ring Before the Ribose
In contrast to purines, pyrimidine biosynthesis involves constructing the nitrogenous base ring before attaching it to the ribose sugar. The pathway initiates with the synthesis of carbamoyl phosphate from glutamine and bicarbonate, catalyzed by carbamoyl phosphate synthetase II, an enzymatic domain of the multifunctional CAD complex. Carbamoyl phosphate reacts with aspartate to form carbamoyl aspartate, which undergoes cyclization and oxidation to yield orotate. Orotate is subsequently combined with PRPP by orotate phosphoribosyltransferase (OPRT) to generate orotidine monophosphate (OMP), which is then decarboxylated to form uridine monophosphate (UMP). UMP serves as the precursor for the synthesis of other pyrimidine nucleotides such as cytidine triphosphate (CTP) and, via the deoxyribonucleotide pathway, thymidine monophosphate (TMP).
Salvage Pathway: Recycling for Cellular Efficiency
The salvage pathway complements both purine and pyrimidine biosynthesis by recycling free nitrogenous bases and nucleosides derived from cellular turnover. Enzymes like hypoxanthine-guanine phosphoribosyltransferase (HGPRT) and thymidine kinase (TK1) play essential roles in this energy-saving route. This pathway is particularly important in tissues with low de novo synthesis capacity, such as the brain. The integration of de novo and salvage pathways, modulated through complex feedback mechanisms and interlinked with metabolic networks like the folate and amino acid pathways, ensures a stable nucleotide pool for the cell's diverse needs.
._1745289520_WNo_676d943.webp)
The de novo pyrimidine and purine synthesis pathways (Villa et al., 2019).
Lifecycle of a Nucleotide: Metabolism and Regulation
Once synthesized, nucleotides undergo dynamic metabolic transformations to fulfill their cellular functions. Nucleotide metabolism includes both anabolic processes for synthesis and catabolic pathways for degradation and recycling.
Purines are catabolized through a well-defined pathway in which AMP and GMP are dephosphorylated and deaminated to form inosine and guanosine, respectively. These nucleosides are further degraded to hypoxanthine and xanthine, which are subsequently oxidized by xanthine oxidase to produce uric acid. While uric acid serves as an antioxidant in plasma, excessive accumulation can lead to gout and kidney stones.
Pyrimidines are degraded in a more energy-efficient manner. Cytidine and uridine are deaminated to form uracil, while thymidine is converted into thymine. These bases are further broken down to β-alanine and β-aminoisobutyric acid, which can enter the tricarboxylic acid (TCA) cycle or be excreted. Key enzymes in these pathways include adenosine deaminase, purine nucleoside phosphorylase, and dihydropyrimidine dehydrogenase.
Regulatory mechanisms ensure a balanced nucleotide pool within the cell. Ribonucleotide reductase controls the reduction of ribonucleotides to deoxyribonucleotides, crucial for DNA replication and repair. Feedback inhibition by end products such as ATP and GTP modulates the activity of key biosynthetic enzymes. Any imbalance in these regulatory systems can result in pathophysiological states, including immunodeficiencies, megaloblastic anemia, or oncogenic transformation.
, salvage _1745283602_WNo_691d702.webp)
Pathways of purine metabolism. Purine metabolism includes de novo synthesis (orange dotted line), salvage pathway (blue dotted line) and catabolic pathway (purple dotted line). (Feng et al., 2022)
Molecular Health Guardians: Nucleotides in Human Health and Disease
Far beyond their genetic roles, nucleotides actively shape human physiology—fueling energy metabolism, enabling immune defense, and supporting brain function. Recent discoveries have uncovered their diverse impacts on health and disease, revealing how imbalances or disruptions in nucleotide metabolism can contribute to a wide range of conditions.
Brain Builders: Nucleotides and Cognitive Function
Nucleotides, particularly cytidine and uridine, are indispensable for neuronal membrane phospholipid synthesis, which is critical for neurodevelopment and synaptic plasticity. These nucleotides act as precursors for cytidine triphosphate (CTP), involved in the biosynthesis of phosphatidylcholine and phosphatidylethanolamine. Research has shown that supplementation with uridine and choline enhances synaptic formation and dendritic spine density, potentially improving cognitive functions in neurodegenerative conditions such as Alzheimer's disease. Additionally, nucleotides support the energy demands of neural cells through ATP, facilitating neurotransmission and long-term potentiation processes essential for learning and memory.
Immunity Engineers: Nucleotides in Immune Function
The immune system is one of the most metabolically active systems in the body, requiring rapid nucleotide synthesis for lymphocyte proliferation and differentiation. De novo purine synthesis is especially vital during T-cell activation, as the need for DNA replication spikes. Moreover, nucleotides like inosine have been found to exert anti-inflammatory effects by modulating cytokine production and inhibiting pro-inflammatory signaling pathways such as NF-κB. Clinical studies indicate that dietary nucleotide supplementation can enhance the function of natural killer cells, increase antibody responses, and improve resistance to infections, especially in immunocompromised individuals and infants.
Cancer Fuel or Foe? Nucleotide Dysregulation in Oncology
Cancer cells exhibit increased nucleotide biosynthesis to support uncontrolled proliferation. Overexpression of enzymes such as ribonucleotide reductase, thymidylate synthase, and dihydrofolate reductase are hallmarks of rapidly dividing tumor cells. Therapeutic agents like 5-fluorouracil (5-FU) and methotrexate target these pathways by mimicking nucleotide structures or inhibiting key enzymes, effectively blocking DNA synthesis. Furthermore, altered nucleotide metabolism contributes to drug resistance and tumor progression, making this pathway a promising focus for novel anticancer strategies. By profiling nucleotide metabolites, clinicians can assess tumor metabolic states and personalize treatment protocols.
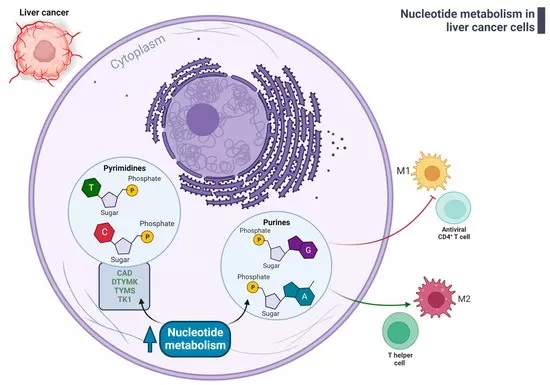
Deregulated nucleotide metabolism in liver cancer cells affects immune microenvironment (Foglia et al., 2023)
Nucleotides in Plants: The Silent Architects of Growth
In the plant kingdom, nucleotides serve not just as genetic blueprints but as regulators of defense, development, and growth. These versatile molecules are at the heart of signaling pathways and biosynthetic networks that help plants adapt to their environment and sustain their vitality.
Signal Messengers: Nucleotides in Plant Immunity
In plants, cyclic nucleotides such as cAMP and cGMP serve as second messengers in defense signal transduction. Upon pathogen attack, these cyclic nucleotides activate a cascade of responses, including calcium influx, reactive oxygen species (ROS) production, and the expression of pathogenesis-related (PR) genes. For instance, cGMP has been shown to regulate stomatal closure, reducing pathogen entry. Additionally, extracellular ATP acts as a danger signal (DAMP) that modulates defense hormone pathways involving jasmonic acid and salicylic acid. Mutants deficient in nucleotide signaling components display compromised resistance, underscoring their role in innate immunity.
Growth Coordinators: Nucleotide Impact on Cell Division
Plant growth and development depend heavily on adequate nucleotide availability to support DNA replication and ribosome biogenesis. Meristematic tissues, which harbor actively dividing cells, exhibit high expression levels of nucleotide biosynthesis genes. These include enzymes like ribose-phosphate pyrophosphokinase and orotate phosphoribosyltransferase. Moreover, nucleotide metabolism is tightly integrated with photosynthetic activity, as ATP and NADPH generated from light reactions feed into biosynthetic pathways. Hormonal signals such as auxins and cytokinins further regulate nucleotide synthesis by modulating gene expression, ensuring that nucleotide supply matches cellular demand during organogenesis.
Everyday Essentials: Nucleotides in Daily Life
Nucleotides are not confined to the microscopic world—they influence aspects of our daily lives in surprising and practical ways. From nutrition and wellness to biotechnology and diagnostics, these molecules quietly but powerfully shape the modern world.
Nutritional Value in Infant Formula and Functional Foods
Nucleotides are now commonly added to infant formulas to replicate the nucleotide content of human breast milk, which supports the development of the gastrointestinal and immune systems. Clinical studies have demonstrated that nucleotide-enriched formulas reduce the incidence of diarrhea and enhance the antibody response to vaccinations. In adults, dietary nucleotides may support liver regeneration, improve recovery from gastrointestinal disorders, and modulate gut microbiota composition. Functional foods and sports supplements containing nucleotides are gaining popularity for their roles in enhancing energy metabolism, reducing fatigue, and supporting tissue repair.
Biotechnology and Diagnostics
Synthetic nucleotides are indispensable in modern biotechnology. They are core components of molecular biology techniques such as polymerase chain reaction (PCR), DNA sequencing, and gene editing systems like CRISPR-Cas9. Modified nucleotides are used to improve the stability and fidelity of DNA polymerases, while fluorescently labeled nucleotides enable real-time tracking of genetic reactions. In diagnostics, nucleotide-based probes and aptamers are employed for the detection of pathogens, genetic mutations, and biomarkers, playing a vital role in personalized medicine and public health surveillance.
Decode Life with MetwareBio: Your Partner in Nucleotide Metabolomics
Understanding the complexity of nucleotide metabolism offers valuable insights into a wide range of biological processes and disease mechanisms. From supporting brain function to fueling cancer cells, nucleotides are at the heart of cellular life. At MetwareBio, our cutting-edge metabolomics platform provides comprehensive profiling of purine and pyrimidine metabolites, enabling researchers to dissect nucleotide pathways with precision. By leveraging high-throughput technologies and pathway-specific panels, we empower scientists and clinicians to explore nucleotide dynamics in health, disease, and therapy. Partner with MetwareBio to unlock the full potential of nucleotide metabolomics and drive your discoveries forward.
References
Traut T. W. (1994). Physiological concentrations of purines and pyrimidines. Molecular and cellular biochemistry, 140(1), 1–22. https://doi.org/10.1007/BF00928361
Lane, A. N., & Fan, T. W. (2015). Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic acids research, 43(4), 2466–2485. https://doi.org/10.1093/nar/gkv047
Feng S., Wu S., Xie F., Yang CS., Shao P. (2022) Natural compounds lower uric acid levels and hyperuricemia: Molecular mechanisms and prospective. Trends in Food Science & Technology, 123:87-102. https://doi.org/10.1016/j.tifs.2022.03.002.
Villa, E., Ali, E. S., Sahu, U., & Ben-Sahra, I. (2019). Cancer Cells Tune the Signaling Pathways to Empower de Novo Synthesis of Nucleotides. Cancers, 11(5), 688. https://doi.org/10.3390/cancers11050688
Foglia, B., Beltrà, M., Sutti, S., & Cannito, S. (2023). Metabolic Reprogramming of HCC: A New Microenvironment for Immune Responses. International journal of molecular sciences, 24(8), 7463. https://doi.org/10.3390/ijms24087463


