Mastering Western Blot: Principles, Workflow and Essential Optimization Strategies
Western Blot (WB) is an indispensable analytical technique widely utilized in molecular biology and biochemistry laboratories to identify specific proteins within complex biological samples. Renowned for its specificity, Western Blot leverages the principle of antigenantibody interactions, providing researchers with critical insights into protein expression, identification, and modifications such as phosphorylation. The application of Western Blot spans numerous fields, including biomedical research, drug discovery, disease diagnosis, and therapeutic target validation. In this article, we thoroughly explore the fundamental principles, standard workflow, and essential optimization strategies to perform effective Western Blot experiments, ultimately enabling precise, reproducible, and reliable results.
Core Principles of Western Blot
Western Blot is essentially an analytical method that combines protein electrophoresis with immunological detection. Proteins extracted from cells or tissues are first separated based on molecular weight by sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDSPAGE). After separation, the proteins are transferred to a solidphase membrane, typically nitrocellulose (NC) or polyvinylidene fluoride (PVDF), where they are immobilized for subsequent analysis.
Once immobilized, target proteins on the membrane are probed using primary antibodies specific to unique protein epitopes. Secondary antibodies conjugated with an enzyme such as horseradish peroxidase (HRP) detect the primary antibodies, allowing visualization through enhanced chemiluminescence (ECL). Western Blot thus enables the detection, semiquantification, and confirmation of protein identity and posttranslational modifications.
Step-by-Step: An Overview of the Western Blot Workflow
Step 1: Protein Extraction
Protein extraction forms the cornerstone of the Western Blot workflow, setting the stage for accurate and reliable downstream analyses. Effective protein extraction requires appropriate lysates designed to disrupt cellular or nuclear membranes without compromising protein integrity. Commonly used lysis buffers include RIPA buffer, NP40 buffer, and ureabased solutions. For instance, RIPA buffer, containing detergents like Triton X100 and SDS, is versatile for extracting both cytoplasmic and nuclear proteins. In contrast, NP40 buffer is gentler, preserving membrane protein structures.
Critically, protein extraction must incorporate protease inhibitors such as Complete™ tablets (Roche) or PMSF to prevent enzymatic degradation, ensuring protein stability and integrity for accurate downstream analysis.
Step 2: Protein Quantification and Sample Preparation
Protein quantification ensures consistent protein loading across gel lanes, essential for reliable comparative analyses. Common quantification methods include Bradford, Lowry, and BCA assays, each varying in sensitivity and compatibility with detergents. Among these, the BCA assay is preferred for its accuracy and stability, facilitating consistent sample loading.
After quantification, protein samples undergo denaturation in loading buffer containing SDS to ensure proteins acquire a uniform negative charge and linear structure, enabling separation by electrophoresis purely based on molecular weight. Reducing agents such as dithiothreitol (DTT) are also employed to break disulfide bonds, maintaining protein linearity and further standardizing migration.
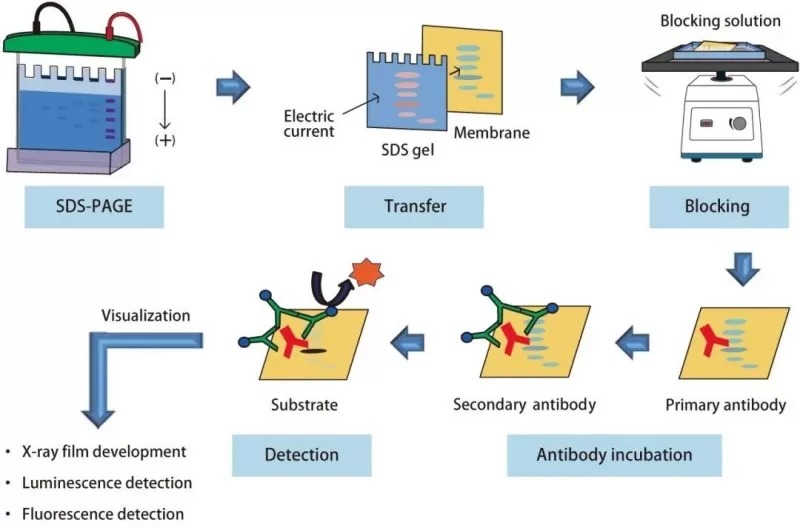
The workflow of western blot experiment
Step 3: Electrophoresis and Protein Separation
SDSPAGE electrophoresis separates proteins based on size through a polyacrylamide gel matrix under an electric field. Initially, proteins migrate through a stacking gel at low voltage to concentrate bands, then enter a resolving gel at higher voltage (120V), clearly separating proteins by molecular weight. Tracking dyes such as bromophenol blue visually indicate progress, preventing the risk of proteins migrating out of the gel.
Step 4: Protein Transfer to Membrane
Following electrophoresis, proteins are transferred electrophoretically onto NC or polyvinylidene fluoride (PVDF) membranes. NC membranes offer high proteinbinding capacities ideal for most applications. In contrast, PVDF membranes are preferred for hydrophobic or high molecular weight proteins due to their superior mechanical strength and protein retention. Typically, proteins are transferred at constant current (200 mA) under cooled conditions (4°C) for 12 hours, ensuring efficient and uniform transfer.
Step 5: Blocking and Antibody Incubation
After transfer, membranes undergo blocking to prevent nonspecific antibody binding. Blocking agents such as 5% skim milk or bovine serum albumin (BSA) are commonly employed. While milkbased blocking agents are effective for routine analyses, BSA is essential for detecting phosphorylated proteins since milk components can interfere with antibodyantigen interactions.
The membranes are then incubated sequentially with primary antibodies targeting the protein of interest and secondary antibodies conjugated to detection molecules like horseradish peroxidase (HRP). Primary antibody dilution is a critical parameter typically ranging from 1:500 to 1:2000, validated through preliminary experiments to optimize signaltonoise ratios. Secondary antibodies amplify signals, allowing sensitive detection through ECL.
Step 6: Detection and Analysis
Protein detection utilizes enhanced chemiluminescence (ECL), a reaction producing detectable light signals. ECL substrates (luminol and peroxidebased) must be freshly prepared and immediately applied to the membrane in darkness to achieve maximum sensitivity. Chemiluminescent signals are captured digitally or on Xray film, followed by densitometric analysis with software tools such as ImageJ or Image Lab, enabling precise semiquantitative comparisons of protein expression levels normalized to housekeeping controls (e.g., βactin or GAPDH).
Common Pitfalls and Troubleshooting Tips
Despite its widespread utility, Western Blot experiments can encounter common challenges affecting data quality:
- High background noise can be mitigated by extending blocking duration or optimizing antibody dilution.
- Nonspecific bands usually result from overly concentrated primary antibodies; diluting antibodies or changing blocking solutions can resolve this.
- Protein degradation and insufficient protein transfer necessitate careful handling, maintaining low temperature throughout preparation, and verifying transfer efficiency using prestained protein markers.
- Keeping membranes hydrated throughout processing prevents protein denaturation and loss of target signals.
Essential Databases to Enhance Experimental Planning
Researchers can significantly enhance their experimental outcomes by utilizing dedicated bioinformatics resources such as BioGPS, the Human Protein Atlas (HPA), UniProt, and PhosphoSitePlus databases. These platforms provide valuable data on protein expression profiles across tissues, known isoforms, and posttranslational modifications, guiding experiment design and interpretation.
Read more
What is Phospholipid? Structure, Functions, and Applications
Unveiling Ornithine: Beyond the Urea Cycle, A Multifaceted Player in Health
Top-Down vs. Bottom-Up Proteomics: Unraveling the Secrets of Protein Analysis
Comprehensive Guide to Basic Bioinformatics Analysis in Proteomics
Strategies for Selecting Key Interacting Proteins in IP-MS Studies


