Using Proteomics to Improve the Drug Development Process
Overview

1. Introduction
Proteomics, the large-scale study of proteins, has emerged as a cornerstone in modern biomedical research and drug development. Proteins, as the functional molecules within cells, provide key insights into biological processes, including disease mechanisms, drug interactions, and therapeutic efficacy. In recent years, proteomics has been leveraged to advance the understanding of diseases at a molecular level, leading to breakthroughs in drug discovery, biomarker identification, and personalized medicine.
Traditional drug development processes often face challenges, including high failure rates, lengthy development timelines, and inadequate understanding of complex disease pathways. Proteomics offers a solution by providing deep insights into the protein networks that underlie disease, enabling the identification of novel drug targets and the development of more effective, tailored therapies.
2. What is Proteomics?
Proteomics refers to the large-scale study of the proteome, which encompasses the entire set of proteins expressed in a cell, tissue, or organism at any given time. Unlike genomics, which focuses on the genetic blueprint, proteomics provides a dynamic and functional perspective, as proteins are the ultimate effectors of cellular processes.
Key technologies used in proteomics include:
- Mass Spectrometry (MS): A critical tool for identifying and quantifying proteins based on their mass-to-charge ratios. MS enables high-throughput analysis of complex protein samples, revealing protein expression patterns, post-translational modifications, and protein-protein interactions.
- Protein Microarrays: Used for profiling protein interactions and detecting biomarkers across different biological conditions.
- 2D Gel Electrophoresis: Allows for the separation and identification of proteins based on their size and charge.
Proteomics differs from genomics and transcriptomics by focusing not on the genetic code or mRNA expression but on the proteins themselves. While genomics reveals potential, proteomics provides the functional insights necessary to understand how genes translate into actionable biological effects.
3. The Role of Proteomics in Drug Development
Proteomics plays a pivotal role in the drug development process, particularly in the identification of novel drug targets and the understanding of disease at a molecular level. By analyzing the proteome of diseased tissues or cell lines, researchers can pinpoint specific proteins or protein networks that contribute to the disease state.
-
Identifying Drug Targets: One of the most significant contributions of proteomics is its ability to uncover drug targets by identifying dysregulated proteins in disease pathways. This enables the development of therapies that can modulate protein activity, whether through small molecules, biologics, or monoclonal antibodies.
-
Understanding Disease Mechanisms: By examining changes in protein expression, post-translational modifications, and protein-protein interactions, proteomics provides insights into the molecular mechanisms driving diseases such as cancer, neurodegeneration, and autoimmune disorders.
-
Biomarker Discovery: Proteomics aids in the identification of biomarkers that are critical for early disease diagnosis, prognostic prediction, and treatment monitoring. These biomarkers are invaluable in the development of personalized medicine, where treatments are tailored to an individual’s protein profile, improving efficacy and minimizing side effects.
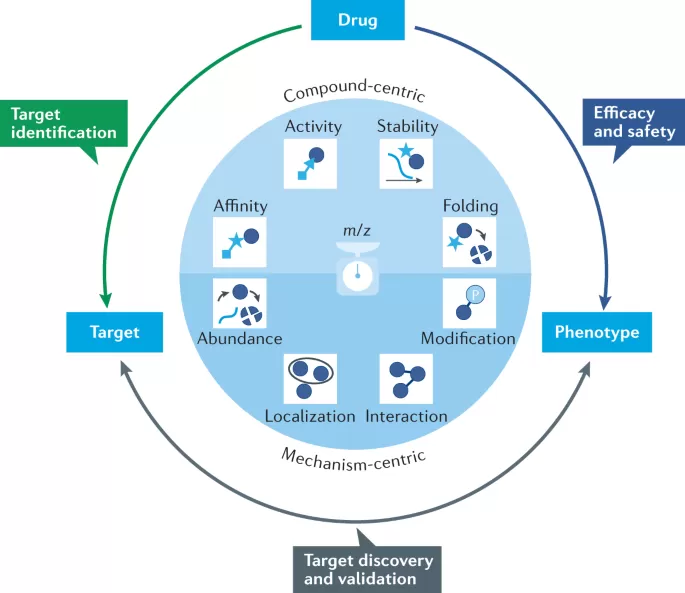
The role of proteomics in drug development (M. et al., 2022)
4. Applications of Proteomics in Drug Development
Target Identification and Validation
Proteomics plays an instrumental role in identifying potential drug targets. By analyzing disease-specific proteomes, researchers can pinpoint proteins that are overexpressed, mutated, or involved in disease pathways. Validating these targets through functional proteomics ensures that they are not only relevant to the disease but also druggable.
Drug Efficacy and Toxicity Studies
In drug development, proteomics helps evaluate both the efficacy and toxicity of drug candidates. By assessing protein expression and post-translational modifications in response to drug treatment, proteomics provides early insights into how a drug interacts with its target and potential off-target effects. This allows researchers to refine compounds before moving into clinical trials, thus accelerating the development process.
Biomarker Discovery
Proteomics enables the identification of biomarkers for disease detection and progression. By profiling the proteomes of patients at different disease stages, researchers can identify proteins that serve as reliable indicators of disease presence or severity. These biomarkers are crucial for diagnostic and monitoring applications in clinical settings, especially in oncology and cardiovascular diseases.
Personalized Medicine
The integration of proteomics with genomics and transcriptomics supports the development of personalized therapies. By analyzing an individual’s protein expression profile, clinicians can select the most effective drugs for a particular patient, thereby enhancing treatment outcomes and minimizing adverse effects.
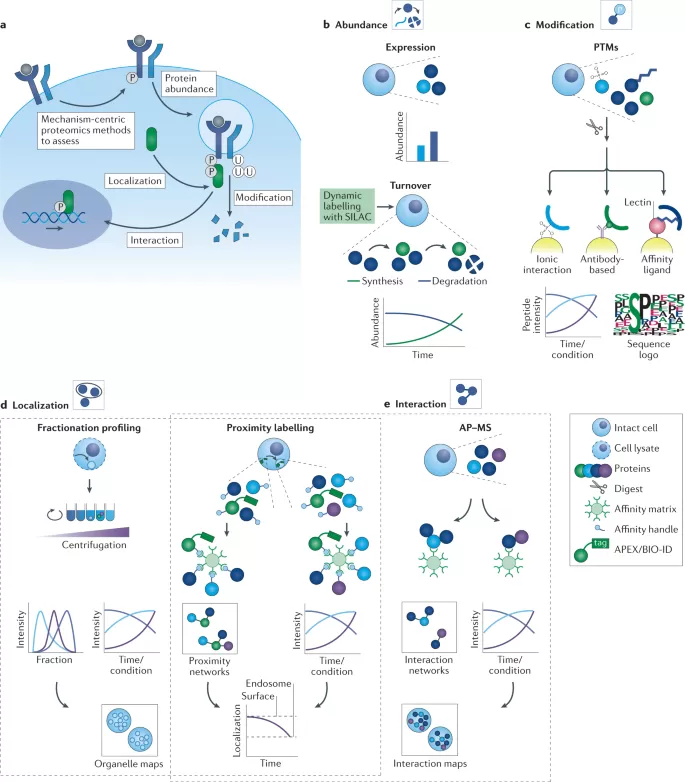
Applications of Proteomics in Drug Development (M. et al., 2022)
5. Case Studies and Real-World Examples
Numerous successful drug development projects have leveraged proteomics to drive breakthroughs. One notable example is the development of targeted cancer therapies, such as HER2-targeted therapies for breast cancer. Proteomic profiling identified HER2 as a key protein in cancer progression, leading to the creation of trastuzumab, a monoclonal antibody that specifically targets HER2.
Another example is proteomics-driven biomarker discovery in Alzheimer's disease. By analyzing the proteomes of brain tissue from Alzheimer's patients, researchers identified proteins associated with amyloid plaque formation, contributing to the development of diagnostic tests and novel therapeutic approaches.
These examples demonstrate how proteomics not only accelerates drug discovery but also reduces the time and cost associated with bringing drugs to market.
6. Challenges and Limitations of Proteomics in Drug Development
Despite its potential, proteomics faces several challenges in drug development:
-
Technical Challenges: The complexity of protein mixtures, the low abundance of certain proteins, and the dynamic nature of protein expression make proteomic analysis highly challenging. The development of more sensitive and accurate mass spectrometry techniques is crucial to overcoming these obstacles.
-
Cost and Resource Requirements: High-throughput proteomic studies are expensive and require significant resources, including specialized equipment, expertise, and computational infrastructure for data analysis.
-
Ethical and Regulatory Considerations: As with any advanced medical technology, proteomics raises ethical concerns regarding the use of biomarkers and patient data. Regulatory bodies must develop frameworks that ensure the safe and ethical use of proteomic data in clinical trials.
7. Future Trends in Proteomics and Drug Development
The future of proteomics in drug development is promising, with several trends poised to drive innovation:
-
Single-Cell Proteomics: Emerging technologies in single-cell proteomics will enable the analysis of protein expression at the individual cell level, providing deeper insights into cellular heterogeneity in diseases such as cancer.
-
AI-Driven Proteomics: The integration of artificial intelligence (AI) and machine learning with proteomic data analysis will enable faster, more accurate interpretation of large datasets, accelerating drug discovery and biomarker identification.
-
Multi-Omics Integration: The future of drug development lies in the integration of genomics, transcriptomics, and proteomics. A holistic view of the molecular landscape will allow for more precise drug targeting and the development of truly personalized medicines.
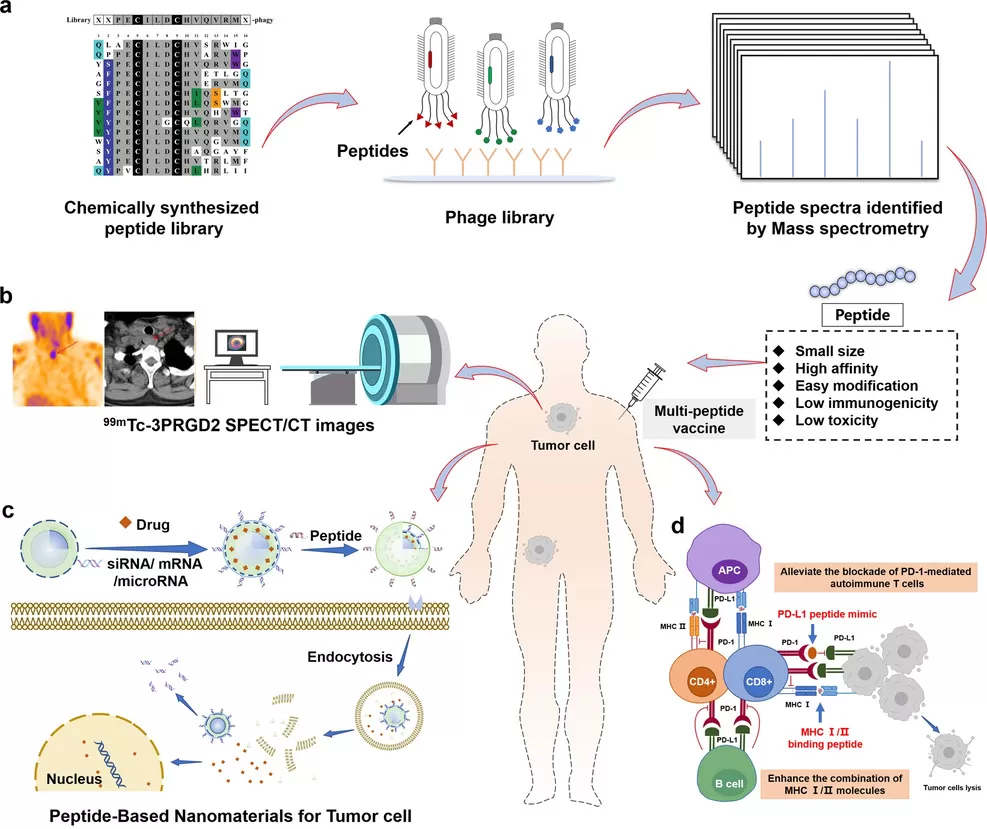
Peptides can be applied in tumor therapy in four main ways (W. et al., 2022)
Proteomics is revolutionizing the drug development process by providing comprehensive insights into disease biology and the identification of novel drug targets. Through biomarker discovery, target validation, and personalized therapies, proteomics is paving the way for more effective and efficient drug development. However, overcoming the technical challenges and addressing the ethical considerations will be key to unlocking the full potential of proteomics in transforming medicine. Continued investment in proteomics technologies and interdisciplinary research will be essential to advancing this field.
8. FAQ Section
Q1: What is proteomics, and how is it used in drug development?
A1: Proteomics is the study of the proteome, focusing on the identification and analysis of proteins in biological systems. It is used in drug development to identify drug targets, understand disease mechanisms, and discover biomarkers for diagnosis and treatment.
Q2: How does proteomics improve the accuracy of drug target identification?
A2: By analyzing protein expression patterns in disease states, proteomics enables the identification of dysregulated proteins that serve as potential drug targets. This allows for more accurate and effective drug development.
Q3: What are the main challenges of using proteomics in drug development?
A3: The main challenges include the complexity of protein mixtures, the high cost of proteomic technologies, and the need for specialized expertise. Additionally, interpreting large-scale proteomic data remains a complex task.
Q4: Can proteomics help in developing personalized medicine?
A4: Yes, proteomics plays a vital role in personalized medicine by providing detailed information about an individual’s protein profile, which can be used to tailor treatments to improve efficacy and minimize side effects.
Contact MetwareBio Today
MetwareBio offers advanced and comprehensive proteomics services, providing everything from sample processing and protein detection to in-depth data analysis and the delivery of high-quality analytical reports. Our data analysis reports encompass various functional annotations and enrichment analyses for differentially expressed proteins, along with protein interaction network and WPCNA (Weighted Protein Co-expression Network Analysis).
References
1. Meissner, F., Geddes-McAlister, J., Mann, M. et al. The emerging role of mass spectrometry-based proteomics in drug discovery. Nat Rev Drug Discov 21, 637–654 (2022). https://doi.org/10.1038/s41573-022-00409-3
2. Wang, L., Wang, N., Zhang, W. et al. Therapeutic peptides: current applications and future directions. Sig Transduct Target Ther 7, 48 (2022). https://doi.org/10.1038/s41392-022-00904-4
Read More
The Fine Art of Protein Methylation: Mechanisms, Impacts, and Analytical Techniques
Maximizing Proteomic Potential: Top Software Solutions for MS-Based Proteomics
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


