Top Mass Spectrometry Instruments Compared: Features, Strengths, and Selection Tips
A mass spectrometer is a sophisticated instrument capable of ionizing samples and analyzing their composition and structure based on the mass-to-charge ratio (m/z). Its core principle involves ionizing the sample, separating ions of different m/z values via electromagnetic fields, and recording the relative abundance of ions using a detector to generate a mass spectrum for analysis. The main components of a mass spectrometer include: 1) Inlet System: Introduces the sample into the instrument, such as via a gas chromatography (GC) or liquid chromatography (LC) interface; 2) Ion Source: Converts sample molecules into charged ions using techniques like electron impact (EI) or electrospray ionization (ESI); 3) Mass Analyzer: Separates ions by m/z, including quadrupoles, ion traps (e.g., linear ion trap, Orbitrap), and time-of-flight (TOF); 4) Detector: Records ion signals and converts them into analyzable mass spectral data. To address diverse analytical challenges, various types of mass spectrometers have been developed. Below are detailed descriptions of several instruments to enhance experimental design and research efficiency.
For a quick comparison of instruments, refer to the Mass Spectrometer Comparison Summary table below.
1. TSQ Quantum Access MAX Triple Quadrupole Mass Spectrometer
The TSQ Quantum™ Access MAX, developed by Thermo Scientific, is a liquid chromatography-tandem mass spectrometry (LC-MS/MS) system with exceptional sensitivity, specificity, and flexibility. It meets both quantitative and qualitative demands for diverse applications.

Thermo Scientific™ TSQ Quantum™ Access MAX Triple Quadrupole Mass Spectrometer
1) Core Features
- Mass range up to m/z 3000
- High-Selectivity Reaction Monitoring (H-SRM)
- Quantitative Enhanced Data-Dependent MS/MS (QED-MS/MS)
- Rapid positive/negative ion mode switching (<25 ms)
- Application-specific software packages
2) Detection Principle
A quadrupole analyzer separates ions based on m/z by exploiting the stability of ion trajectories in oscillating electric fields. A quadrupole consists of four parallel metal rods. Opposing rod pairs are connected electrically: one pair applies radiofrequency (RF) voltage, and the other applies direct current (DC) voltage. For a given RF/DC combination, only ions with specific m/z values achieve stable trajectories and reach the detector. By varying the voltages, ions of different m/z are sequentially transmitted.
The triple quadrupole configuration comprises three quadrupoles (Q1, Q2, Q3). Q1 and Q3 are standard quadrupoles, while Q2 (the collision cell) uses RF-only potentials. In the TSQ system, Q2 has a square profile with rods bent at 90° to reduce instrument footprint and noise. Precursor ions selected by Q1 undergo collision-induced dissociation (CID) in Q2, and product ions are analyzed by Q3.
3) Scanning Modes
- Q1MS/Q3MS: Single-stage mass analysis equivalent to a single quadrupole.
- Product Ion Scan: Fragments selected precursor ions in Q2 and scans product ions in Q3.
- Precursor Ion Scan: Detects all precursors generating a specific product ion.
- Neutral Loss Scan: Identifies ions losing a neutral fragment of defined mass.
- Data-Dependent Scan: Triggers MS/MS based on precursor intensity.
- SRM/H-SRM: Quantitative analysis with high selectivity.
- QED-MS/MS: Combines quantification and structural confirmation.
2. Orbitrap Fusion Lumos Tribrid Mass Spectrometer
The Orbitrap Fusion Lumos Tribrid (Thermo Scientific) integrates quadrupole, Orbitrap, and linear ion trap (LIT) analyzers, offering ultrahigh resolution, sensitivity, and versatile fragmentation for proteomics, metabolomics, and biopharmaceutical research.

Orbitrap Fusion Lumos Tribrid Mass Spectrometer
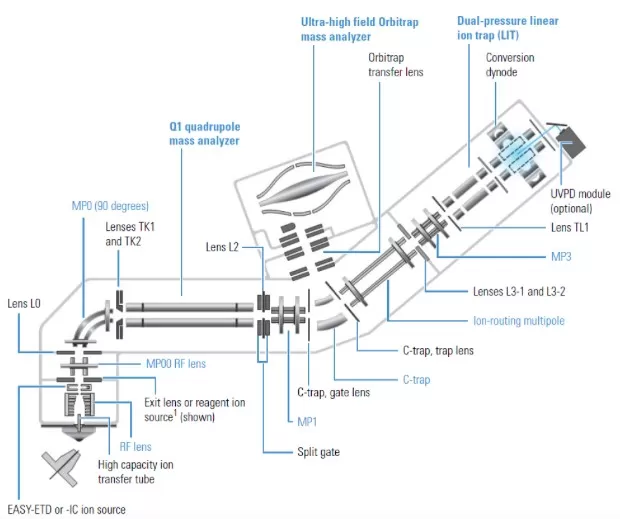
Schematic structure of Orbitrap Fusion Lumos Mass Spectrometer
1) Ion Source
Atmospheric pressure ionization (API) sources include H-ESI, APCI, and APPI. A secondary ion source (RIS) enhances flexibility.
2) Ion Optics
Components such as MP00, MP0, and MP1 ion optics guide ions through the system. The C-trap accumulates and cools ions before injection into the Orbitrap or LIT.
3) Mass Analyzers
Quadrupole (Q1): Filters ions by m/z.
Orbitrap: Measures m/z via image current detection and Fourier transform.
Linear Ion Trap (LIT): Enables MSⁿ fragmentation.
4) Detection System
Dual conversion dynodes and an electron multiplier ensure high signal-to-noise ratios.
5) Scanning Modes
- Full MS Scan: Detects ions across a defined mass range.
- MS²/MSⁿ: Multi-stage fragmentation (CID, HCD, ETD, UVPD).
- SIM: Monitors predefined ions.
3. Agilent 6540 UHD Quadrupole Time-of-Flight (Q-TOF) Mass Spectrometer
The Agilent 6540 UHD Q-TOF combines quadrupole mass filtering with TOF analysis for high-resolution MS/MS. Key features include Jet Stream ESI, ion beam compression (IBC), and enhanced mass accuracy.

Agilent 6540 UHD Q-TOF Mass Spectrometer
1) Structure
- Ion Source: Jet Stream ESI enhances sensitivity.
- Ion Optics: Sampling cone, octopole, and lenses focus ions.
- Quadrupole: Filters precursor ions.
- TOF Analyzer: Measures flight time for m/z determination.
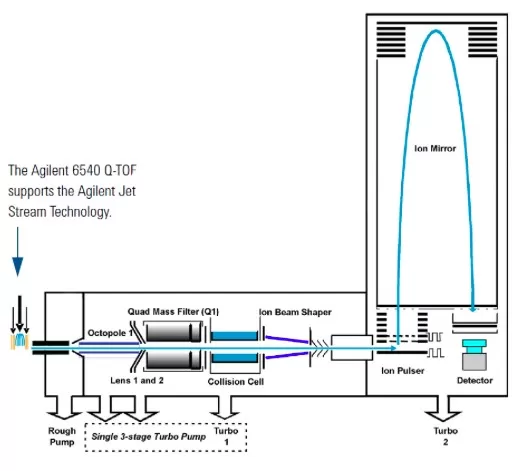
Diagram of the structure of the Agilent 6540 UHD QTOF MS
2) Modes
TOF Mode: Full mass range analysis.
Auto MS/MS: Triggers fragmentation based on user criteria.
Targeted MS/MS: Analyzes predefined ions for high-sensitivity detection.
4. Q Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer
The Q Exactive series (Thermo Scientific) merges quadrupole selectivity with Orbitrap high-resolution detection. Applications include proteomics, metabolomics, and environmental analysis.

Q Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer
1) Advantages
Resolution up to 140,000.
Rapid polarity switching (1 second).
Advanced API sources (ESI, APCI, APPI).
2) Structure
Components include an S-lens, quadrupole, C-trap, HCD cell, and Orbitrap analyzer.
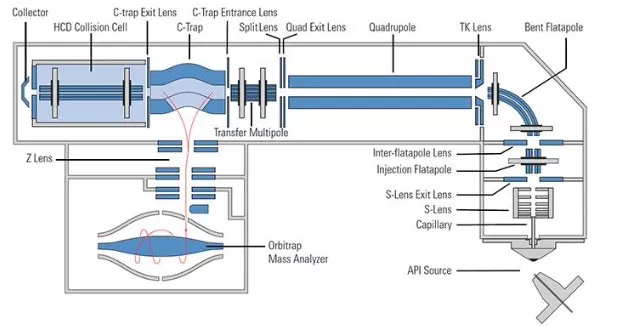
Q Exactive Hybrid Quadrupole Orbitrap Mass Spectrometer Structure
5. Q Exactive Plus Hybrid Quadrupole-Orbitrap Mass Spectrometer
An upgraded version of the Q Exactive, the Q Exactive Plus offers enhanced resolution (up to 280,000) and advanced quantitative modes (PRM, DIA).

Q Exactive Plus Hybrid Quadrupole-Orbitrap Mass Spectrometer
1) Key Features
Advanced Quadrupole Technology (AQT) for improved selectivity.
High sensitivity (500 fg S/N 100:1).
2) Structure of the Q Exactive Plus Hybrid Quadrupole-Orbitrap Mass Spectrometer
Similar to the Q Exactive mass spectrometer, the Q Exactive Plus consists of six main components: an ion source, a mass-resolving injection flatapole, a quadrupole mass filter for precursor ion selection, a curved linear ion trap (C-trap) for short-term storage, a collision cell, and an Orbitrap analyzer for Fourier transform mass spectrometry (FTMS).
In the Q Exactive Plus, the sample is first introduced into the atmospheric pressure ionization (API) source. The injection flatapole transfers ions to the quadrupole while performing a rough pre-filtering based on the mass-to-charge ratio (m/z). The quadrupole then transmits and filters ions according to their m/z values, after which the ions are transferred to the C-trap. In the C-trap, ions are accumulated and their energy is dissipated through collision with buffer gas. Subsequently, the ions are injected into the Orbitrap analyzer to generate the mass spectrum.
If MS/MS experiments or all-ion fragmentation (AIF) are required, ions are directed from the C-trap to the higher-energy collision dissociation (HCD) cell for fragmentation. The resulting fragment ions are then returned to the C-trap and injected into the Orbitrap analyzer for detection.
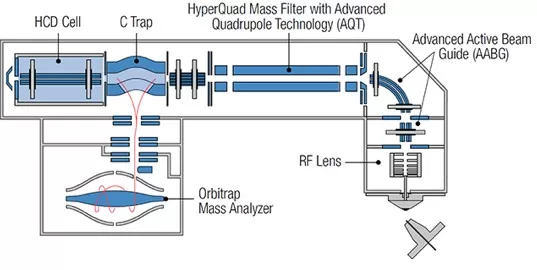
Q Exactive Plus Hybrid Quadrupole Orbitrap Mass Spectrometer Schematic Structure
Mass Spectrometer Comparison Summary Table
|
Instrument |
Mass Analyzer Type |
Key Features |
Strengths |
Limitations |
Best Use Cases |
|
TSQ Quantum Access MAX |
Triple Quadrupole |
H-SRM, QED-MS/MS, fast polarity switching |
High sensitivity and selectivity for quantification; rugged LC-MS/MS |
Lower resolution; less suited for unknowns |
Targeted quantification, clinical assays, environmental monitoring |
|
Orbitrap Fusion Lumos |
Quadrupole + Orbitrap + LIT |
Ultrafast MSn, multiple fragmentation modes, ultrahigh resolution |
Versatile; excellent structural analysis; flexible scan modes |
Complex operation; high cost |
Advanced proteomics, PTMs, metabolomics, drug discovery |
|
Agilent 6540 UHD Q-TOF |
Quadrupole + TOF |
Jet Stream ESI, high mass accuracy, Auto MS/MS |
Good resolution; accurate mass; fast MS/MS |
Slightly lower sensitivity vs. Orbitrap |
Small molecule ID, metabolomics, fast screening |
|
Q Exactive |
Quadrupole + Orbitrap |
Resolution up to 140,000, HCD fragmentation, SIM mode |
Excellent for both quant and ID; high resolution |
No MSn capability; mid-range speed |
Label-free proteomics, metabolomics, complex mixture analysis |
|
Q Exactive Plus |
Quadrupole + Orbitrap |
Higher resolution (up to 280,000), PRM, DIA |
Enhanced quantification; better dynamic range |
Still lacks MSn (vs. Fusion) |
Quantitative proteomics, DIA workflows, biomarker discovery |
How to Choose the Right Mass Spectrometer
To select the most suitable instrument, consider the following factors:
- If your focus is on high-throughput quantification (e.g., clinical samples, targeted small molecule analysis), the TSQ Quantum Access MAX is ideal due to its robustness and SRM capabilities.
- For deep proteome coverage, PTM mapping, or multiple fragmentation strategies, the Orbitrap Fusion Lumos offers unmatched versatility and resolution.
- If speed and high mass accuracy are key, especially for unknown compound screening or metabolomics, go with the Agilent 6540 Q-TOF.
- For balanced performance in both qualitative and quantitative workflows, Q Exactive is a solid choice, especially if MSⁿ isn’t required.
- If you need high-resolution quantification with better dynamic range and PRM/DIA options, the Q Exactive Plus gives you an upgrade without moving to a full tribrid system.
Conclusion
Mass spectrometers ionize samples, separate ions by m/z, and detect signals to determine composition, structure, and quantity. Their high sensitivity, resolution, and versatility make them indispensable in chemistry, biology, environmental science, and beyond. Future advancements in miniaturization and automation will expand their role in clinical diagnostics and other fields.


