TMT Quantitative Proteomics: A Comprehensive Guide to Labeled Protein Analysis
What is Labeled Quantitative Proteomics?
Labeled quantitative proteomics is a sophisticated analytical approach that utilizes isotope labeling to enable accurate quantification of proteins within complex biological samples. This method involves the strategic incorporation of isotope labels—usually stable isotopes—into the proteins or peptides during sample preparation or immediately following enzymatic digestion. Once the samples are labeled and mixed, they undergo proteolytic digestion, typically using enzymes like trypsin, which cleave the proteins into manageable peptides. The resulting peptide mixtures are then analyzed using mass spectrometry. Because the isotope-labeled peptides exhibit distinct mass-to-charge ratios, they can be differentiated during analysis, even when they are present in the same sample. This enables precise quantification of protein abundance, as the relative intensities of the labeled peptides provide insights into the levels of specific proteins across different conditions or time points.
Methods used in Labeled Quantitative Proteomics
In this approach, various labeling techniques can be employed, such as Stable Isotope Labeling with Amino acids in Cell culture (SILAC), Tandem Mass Tags (TMT), or Isobaric Tags for Relative and Absolute Quantification (iTRAQ). Each of these techniques has its own methodology for introducing labels, allowing researchers to select the most appropriate method based on the specific requirements of their study.
SILAC (Stable Isotope Labeling with Amino Acids in Cell Culture) is one of the most prominent techniques employed for labeling during the sample stage. The core principle of SILAC entails the addition of isotopically labeled essential amino acids during the cell culture phase. When the proportion of these stable isotope-labeled amino acids reaches over 95%, different cell populations that have been labeled with distinct isotopes are mixed in equal ratios. This mixture is then subjected to protein extraction, enzymatic digestion, and subsequent analysis via mass spectrometry.While SILAC is a powerful tool for quantitative proteomics, it is primarily applicable to live cultured cells, which can limit its broader utility in proteomic studies. Additionally, the inherent complexity of MS1 spectra often restricts the number of samples that can be effectively analyzed in a single experiment.
To address these limitations, researchers frequently turn to other labeling techniques, one of which is TMT (Tandem Mass Tags). TMT allows for multiplexing of samples, enabling simultaneous analysis of multiple samples within a single mass spectrometry run. TMT (Tandem Mass Tags) and iTRAQ (Isobaric Tags for Relative and Absolute Quantification) are both chemical labeling methods that operate on similar principles. The primary difference between the two lies in their respective manufacturers. TMT, developed by Thermo Fisher, offers a broader range of labeling options, including 6-plex, 10-plex, 16-plex, and 18-plex configurations, thereby extending its applications across various proteomic studies.This capability significantly enhances throughput and reduces variability, making it an invaluable approach in quantitative proteomics. In the following sections, we will delve deeper into the TMT methodology, exploring its principles, applications, and advantages in the field of proteomics.
![Schematic Diagram of Labeled and Label-free Quantitative Proteomics Methods [1] Schematic Diagram of Labeled and Label-free Quantitative Proteomics Methods [1]](https://www.metwarebio.com/uploads/202410/figure 1 TMT Quantitative Proteomics A Comprehensive Guide to Labeled Protein Analysis_1729061341_WNo_749d635.webp)
Reagents of TMT Quantitative Proteomics
![Structural Design of TMTpro Labeling Reagents [2] Structural Design of TMTpro Labeling Reagents [2]](https://www.metwarebio.com/uploads/202410/figure 2 Structural Design of TMTpro Labeling Reagents [2]_1729061370_WNo_738d342.webp) The chemical structure of TMT reagents comprises three essential components: a reporter group, a balance group, and a reactive group. Each TMT label maintains a consistent total molecular weight, while the reporter groups incorporate different isotopes, which lead to variations in molecular weight. The reactive group is specifically designed to interact with the amino group at the N-terminus of the peptide or with reducing cysteine residues, thereby irreversibly labeling all trypsin-digested peptides. This specificity ensures that the labeled peptides can be accurately tracked and quantified. Meanwhile, the balance group, paired with various reporter groups, guarantees that identical peptides from different sources exhibit the same mass-to-charge ratio during the MS1 analysis. This feature is crucial for reliable quantification, as it allows for the differentiation of peptide origins in the second stage of mass spectrometry.
The chemical structure of TMT reagents comprises three essential components: a reporter group, a balance group, and a reactive group. Each TMT label maintains a consistent total molecular weight, while the reporter groups incorporate different isotopes, which lead to variations in molecular weight. The reactive group is specifically designed to interact with the amino group at the N-terminus of the peptide or with reducing cysteine residues, thereby irreversibly labeling all trypsin-digested peptides. This specificity ensures that the labeled peptides can be accurately tracked and quantified. Meanwhile, the balance group, paired with various reporter groups, guarantees that identical peptides from different sources exhibit the same mass-to-charge ratio during the MS1 analysis. This feature is crucial for reliable quantification, as it allows for the differentiation of peptide origins in the second stage of mass spectrometry.
![Schematic Diagram of the TMT Quantitative Proteomics Principle [3] Schematic Diagram of the TMT Quantitative Proteomics Principle [3]](https://www.metwarebio.com/uploads/202410/figure 3 Schematic Diagram of the TMT Quantitative Proteomics Principle [3]_1729061431_WNo_319d651.webp)
Principles of TMT Quantitative Proteomics
The fundamental principle of TMT involves utilizing multiple isotopic labeling reagents to tag the N-termini or lysine side chains of peptide segments derived from different samples post-proteolysis. After labeling, the peptides are mixed for mass spectrometric analysis. Because the labeled peptides share identical molecular weights, they are represented as a single peak in the MS1 analysis. When this peak is selected for fragmentation, each isotopic label produces a unique reporter ion spectrum in the subsequent MS2 analysis. This allows for relative quantification of proteins by comparing the intensities of the reporter ions present in the MS/MS spectra.
Experimental Workflow of TMT Quantitative Proteomics
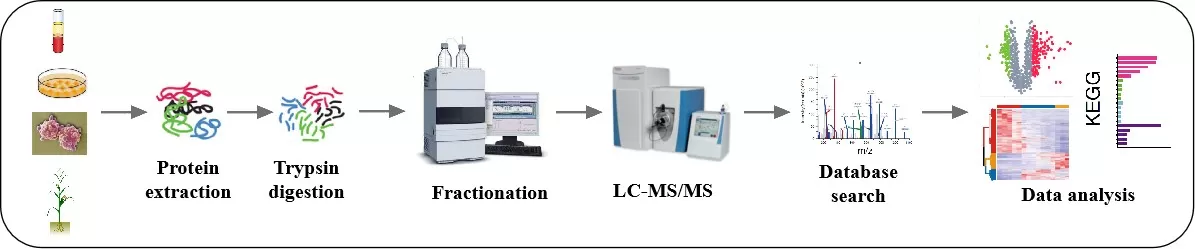
In the workflow of TMT quantitative proteomics, after protein extraction and enzymatic digestion, isotopic labels are employed to tag peptide segments from various samples. Following this, the labeled peptides are combined for analysis. Given the significant amount of information contained within the mixed peptides, it is essential to fractionate the mixture via chromatography into distinct fractions. Each fraction undergoes mass spectrometric analysis, allowing for comprehensive capture of protein information. The greater the number of fractions produced, the deeper the protein coverage obtained, ultimately enhancing the study's depth and accuracy.
Advantages and Limitations of TMT Quantitative Proteomics
One of the primary advantages of TMT quantitative proteomics is its ability to mix and analyze all sample peptides simultaneously, resulting in heightened accuracy and stability in quantification. The fractionation of mixed peptides further enhances sensitivity, enabling the detection of low-abundance proteins that might otherwise remain undetected. However, TMT does have limitations; specifically, the finite number of available tags restricts the number of samples that can be labeled in a single batch, which can introduce batch effects in subsequent experiments. As a result, TMT is best suited for studies involving moderate to low sample sizes and may be less effective for large cohort research, where a greater number of samples is required.
[1] Dominik A Megger, Wael Naboulsi, Helmut E Meyer, Barbara Sitek.Proteome Analyses of Hepatocellular Carcinoma.J Clin Transl Hepatol.2014 Mar;2(1):23-30.
[2] ThermoFisher.Tandem Mass Tag (TMT) Systems.https://www.thermofisher.cn
/cn/zh/home/life-science/protein-biology/protein-mass-spectrometry-analysis/protein-quantitation-mass-spectrometry/tandem-mass-tag-systems.html
[3] Roberto Giambruno 1, Marija Mihailovich 1, Tiziana Bonaldi.Mass Spectrometry-Based Proteomics to Unveil the Non-coding RNA World.Front Mol Biosci.2018 Nov 8:5:90.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


