How the TM6SF2-E167K Variant Drives Liver Disease: A Lipidomics Study
We are excited to highlight a groundbreaking study published in Hepatology, titled Human-induced pluripotent stem cell–based hepatic modeling of lipid metabolism–associated TM6SF2-E167K variant. This open-access research utilized quantitative lipidomics and transcriptomics to investigate how the TM6SF2-E167K genetic mutation disrupts hepatic lipid metabolism, contributing to metabolic dysfunction-associated steatotic liver disease (MASLD). Leveraging MetwareBio’s advanced lipidomics platform, the study identified critical lipid signatures linked to endoplasmic reticulum (ER) stress, mitochondrial dysfunction, and impaired lipid secretion. The findings underscore the value of lipidomics in unraveling complex metabolic disorders and offer new therapeutic insights.
TM6SF2-E167K: Insights from iPSC Models in Liver Disease
Chronic liver disease (CLD) and its progression to conditions like MASLD account for over 2 million deaths annually. Genetic variants, such as the TM6SF2-E167K mutation, are strongly associated with lipid accumulation, inflammation, and liver fibrosis. However, conflicting results from animal models have hindered progress. This study addresses this gap by leveraging **human-induced pluripotent stem cells (iPSCs) to create a physiologically relevant liver disease model. By integrating lipidomics and transcriptomics, the researchers dissected the molecular mechanisms of TM6SF2-E167K, mapping lipid class alterations and uncovering mechanistic links between lipid metabolism dysregulation, cellular stress, and disease, thus providing a blueprint for studying genetic contributions to metabolic disorders.
TM6SF2-E167K Triggers Lipid Accumulation and ER Stress
Using iPSC-derived hepatocytes, the team found that the E167K mutation reduced TM6SF2 protein levels by 50%, leading to intracellular lipid droplet buildup (2.5-fold increase) and impaired VLDL secretion. Lipidomics analysis revealed elevated triglycerides (TGs), phospholipids (PC, PE), and sphingolipids—key markers of lipotoxicity. These changes correlated with ER stress markers (HSPA5, ATF4) and mitochondrial dysfunction, mirroring phenotypes seen in human MASLD patients.
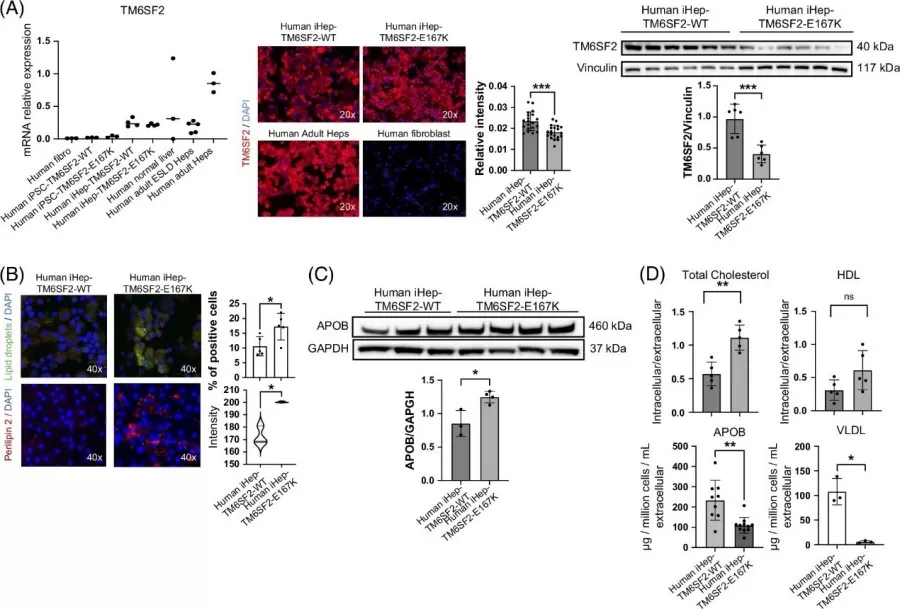
The TM6SF2-E167K mutation results in loss of function and alters lipid metabolism in human iHep.
Transcriptomics and Lipidomics Expose Metabolic Rewiring
Global transcriptomics identified 153 upregulated genes involved in fatty acid oxidation (e.g., PPARGC1A, ACOX1) and cholesterol transport. Complementary lipidomics profiling quantified over 20 lipid classes, showing significant increases in bile acids (e.g., taurolithocholic acid) and free fatty acids. This dual-omics approach highlighted how TM6SF2-E167K reshapes lipid synthesis and degradation pathways, driving disease progression.
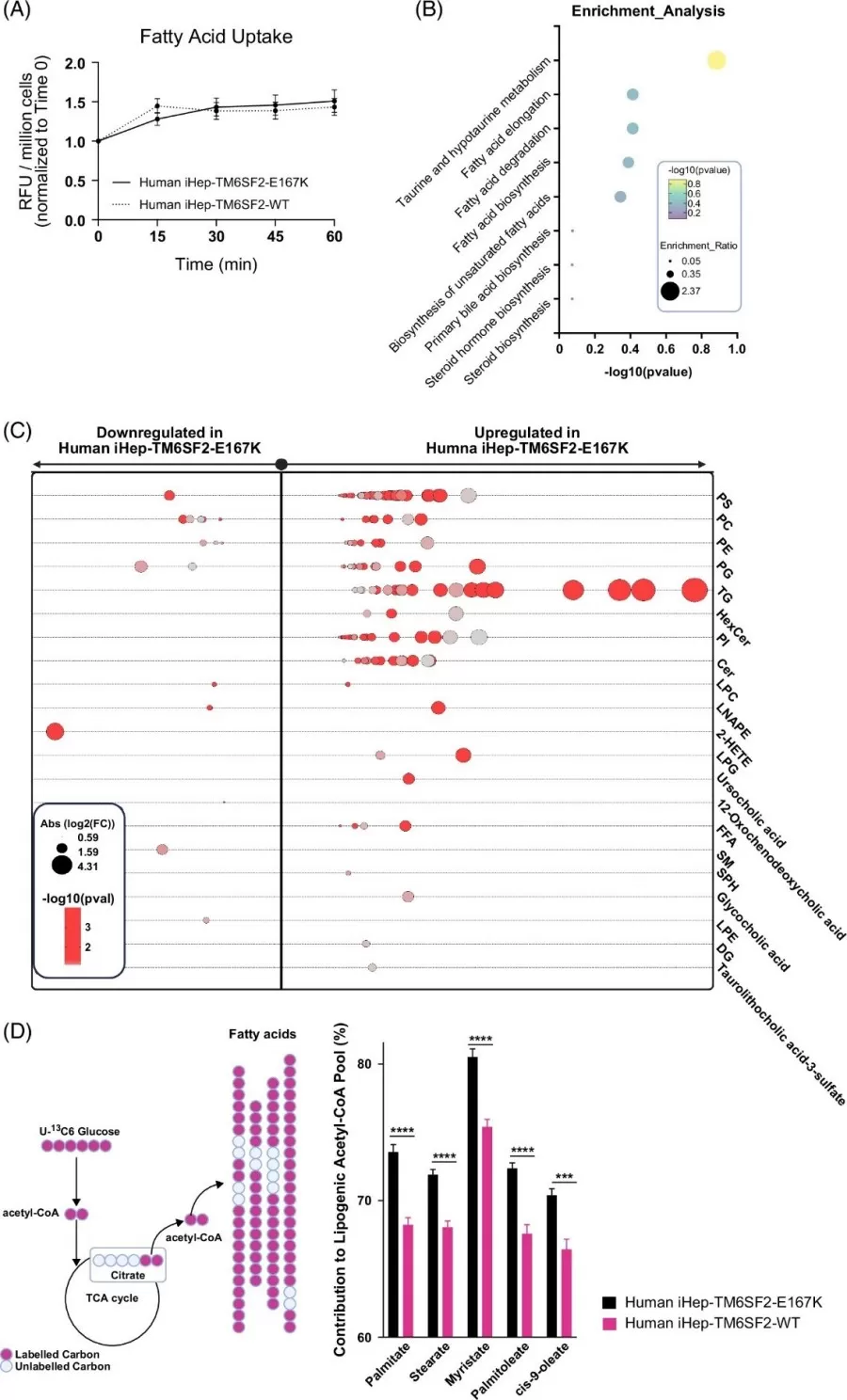
The TM6SF2-E167K variant modifies lipid metabolism in human iHeps.
Therapeutic Rescue via ER Stress Alleviation
Treating mutant iHeps with 4-phenylbutyric acid (4PBA), a protein-folding chaperone, reduced ER stress markers by 40% and restored VLDL secretion. Lipidomics confirmed normalized phospholipid levels, underscoring the potential of targeting protein misfolding to reverse lipid metabolic defects.
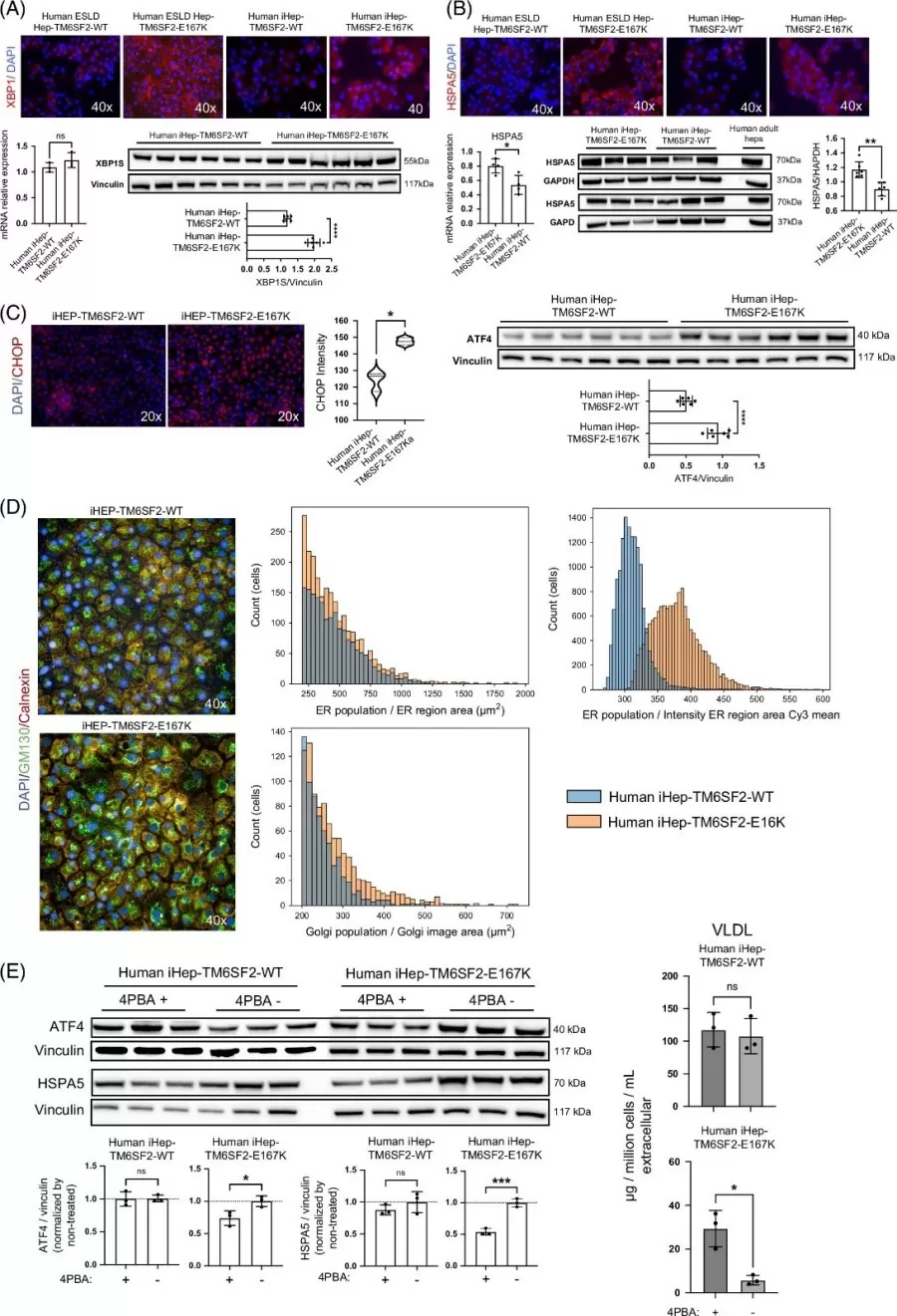
TM6SF2-E167K mutation is related to ER stress.
Leveraging Multi-Omics for Deeper Insights into Disease Mechanisms
This study exemplifies how multi-omics integration—particularly lipidomics—unlocks mechanistic insights into genetic liver diseases. By mapping lipid class alterations and ER stress pathways, the authors validated TM6SF2-E167K as a driver of MASLD and demonstrated the utility of iPSC models in drug discovery. Compared to prior rodent studies, this human-centric approach resolves inconsistencies and provides clinically relevant biomarkers.
Lipidomics emerges as a critical tool for dissecting complex metabolic disorders. Its ability to quantify hundreds of lipids with high precision enables researchers to identify subtle yet pathogenic shifts in lipid homeostasis—a capability unmatched by genomics alone. Future studies could expand this framework to explore lipid-immune crosstalk or screen small molecules targeting lipid-associated stress pathways.
Unlock Precision Lipidomics with MetwareBio
At MetwareBio, we specialize in high-resolution lipidomics services that empower researchers to decode complex metabolic disorders. Our LC-ESI-MS/MS platform delivers:
- Comprehensive Coverage: Quantify 4,000+ lipids across 51 classes.
- High Sensitivity: Detect low-abundance species critical for mechanistic studies.
- End-to-End Expertise: From experimental design to bioinformatics, we ensure actionable insights.
Why Choose MetwareBio?
Cutting-Edge Technology: State-of-the-art instrumentation validated by publications in top journals.
Customized Workflows: Tailored protocols for cells, tissues, or biofluids.
Global Collaboration: Trusted by leading labs worldwide for reproducible, high-impact data.
Uncover the lipid drivers of disease. Partner with MetwareBio today.
Reference
Faccioli LAP, Sun Y, Animasahun O, et al. Human-induced pluripotent stem cell-based hepatic modeling of lipid metabolism-associated TM6SF2-E167K variant. Hepatology. Published online August 27, 2024. doi:10.1097/HEP.0000000000001065
Read more


