Spatial Metabolomics Reveals How Tirzepatide Inhibits Colorectal Cancer via Glucose Metabolic Reprogramming
We are excited to share a recent publication in Advanced Science titled “The Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide Attenuates Colon Cancer Development by Regulating Glucose Metabolism.” In this study, the authors employed spatial metabolomics services provided by MetwareBio to explore the metabolic mechanisms by which tirzepatide (TZP)—a dual GIP and GLP-1 receptor agonist initially approved for type 2 diabetes—exerts potent anti-tumor effects in colorectal cancer (CRC). By integrating high-resolution spatial metabolite profiling with molecular validation, the researchers discovered that TZP disrupts tumor glucose metabolism by inhibiting glycolysis and the tricarboxylic acid (TCA) cycle, leading to energy deprivation in CRC cells and tumor growth suppression. This work not only reveals a novel therapeutic application for TZP but also underscores the power of spatial metabolomics in identifying metabolism-based targets for cancer therapy.
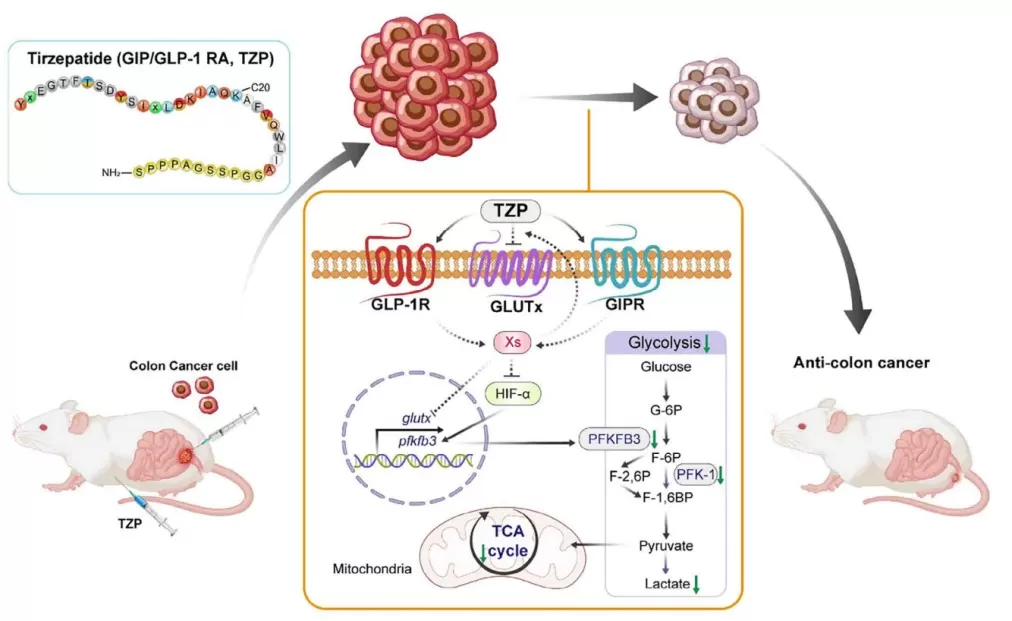
A proposed model of how TZP-mediated dysregulation of glucose metabolism contributes to CRC progression
Exploring the Diabetes–Cancer Connection Through the Lens of Metabolism
Colorectal cancer (CRC) is among the top causes of cancer-related mortality worldwide. Mounting evidence shows that type 2 diabetes (T2DM) significantly increases CRC risk, likely due to shared risk factors (e.g., obesity, age, poor diet) and metabolic dysregulation, such as elevated glucose and insulin levels.
Tirzepatide (TZP) is a novel dual GIPR/GLP-1R agonist developed for managing T2DM. While widely recognized for its efficacy in improving glycemic control and weight loss, this study investigates whether TZP could also suppress cancer growth by targeting glucose metabolism, which is often hijacked by tumor cells to support rapid proliferation—a phenomenon known as the Warburg effect.
TZP Inhibits CRC Cell Growth and Induces Apoptosis, Independent of Glucose Levels
To explore the anti-tumoral potential of TZP in vitro, the researchers first conducted dose-response experiments using the murine colon cancer cell line MC38, identifying 5–50 μM as an effective range that inhibited proliferation without toxicity to normal hepatocytes (BNL CL.2). Similar anti-proliferative and anti-migratory effects were observed in human CRC cell lines HCT116 and RKO, as assessed by transwell migration assays and wound healing assays. Critically, apoptosis was significantly induced in MC38 cells, as confirmed via Annexin V/PI flow cytometry and TUNEL assays, with the effects being independent of extracellular glucose levels (2 mM vs. 33 mM glucose). This indicates that TZP’s antitumor efficacy does not depend on hyperglycemic stress, making it a promising agent even for non-diabetic CRC contexts.
TZP Suppresses Tumor Growth in Diabetic and Non-Diabetic Mouse Models
To validate the in vitro findings in vivo, the study employed multiple murine colorectal cancer models, including orthotopic CRC implantation in T1DM mice (induced with streptozotocin, STZ) and subcutaneous CRC models in T2DM mice (induced by high-fat diet plus STZ), as well as normoglycemic controls.
In all models, daily intraperitoneal injections of TZP (50 nmol/kg for 21 days) resulted in a significant reduction in tumor volume and weight, regardless of diabetic status. Notably, TZP did not significantly alter blood glucose levels in T1DM mice, reinforcing that its anticancer effect is uncoupled from glycemic control. Furthermore, TZP increased survival time in CRC-bearing T1DM mice, highlighting its potential life-prolonging benefit.
Spatial Metabolomics Reveals Decreased Glycolytic Activity in Tumor Tissues
To dissect the metabolic basis of TZP's antitumor activity, the study leveraged spatial metabolomics, performed at MetwareBio, using MALDI-MSI (Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging). This allowed for high-resolution metabolic profiling directly in tissue sections of CRC-bearing mice.
The analysis revealed that TZP-treated tumor regions exhibited profound reductions in glycolysis-related metabolites, including D-glucose, phosphoenolpyruvate, glycerophosphoric acid, and 2′-deoxyinosine. Overall, 541 metabolites were significantly downregulated, while only 4 were upregulated. KEGG pathway enrichment confirmed suppression of glycolysis and TCA cycle-related pathways, indicating a global metabolic reprogramming effect induced by TZP.
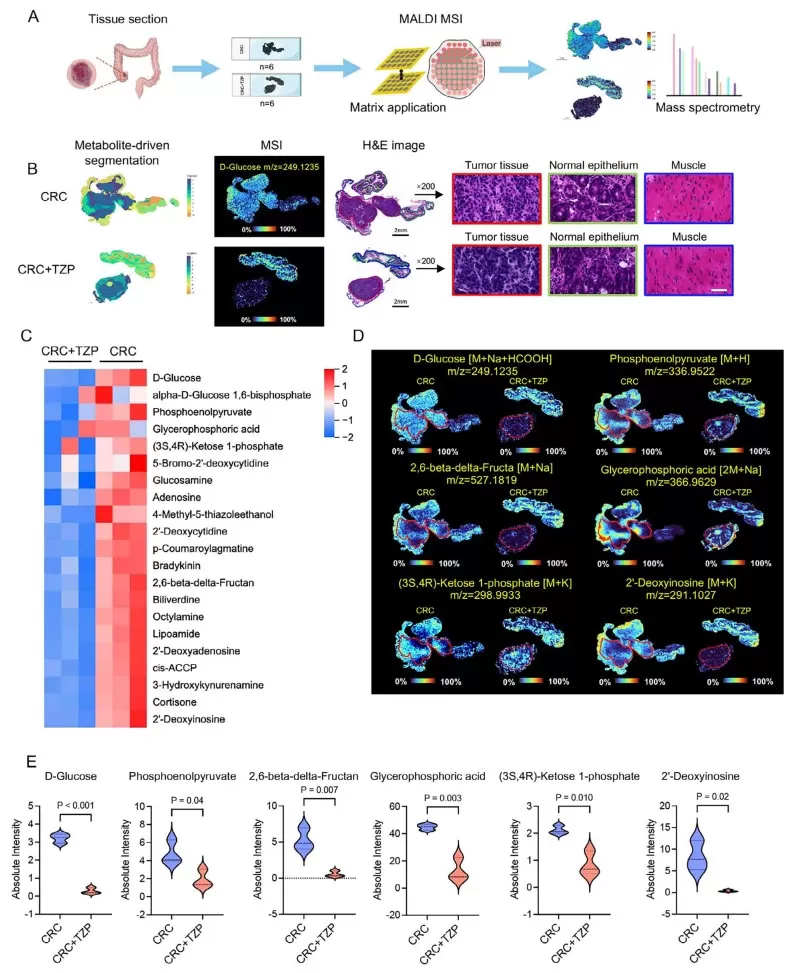
Spatial metabolomics profiled shows that TZP reduced glycolysis in the colon cancer tissues.
TZP Disrupts Glycolytic Flux by Targeting the HIF-1α–PFKFB3–PFK-1 Axis
Mechanistically, the study found that TZP disrupted glycolysis through post-translational regulation of HIF-1α, a central transcription factor activated under hypoxia that regulates metabolic genes. Western blotting and immunohistochemistry showed that TZP treatment reduced HIF-1α expression, inhibited its nuclear translocation, and enhanced its ubiquitination and proteasomal degradation.
Downstream, this led to suppressed expression and activity of two rate-limiting glycolytic enzymes, PFKFB3 and PFK-1, validated via qPCR, ELISA, and enzyme activity assays. The reduction in glycolytic flux was further confirmed by Seahorse XF glycolysis stress tests, which showed decreased extracellular acidification rate (ECAR). Moreover, stable isotope-resolved metabolomics using [U-13C6] glucose showed that TZP blocked the conversion of glucose to TCA intermediates, such as citrate and malate, illustrating dual inhibition of glycolysis and oxidative metabolism.
A Paradigm Shift: Metabolic Reprogramming as a New Anti-Cancer Strategy
This study marks a significant innovation in the field of oncology by demonstrating that tirzepatide (TZP), a dual GIP/GLP-1 receptor agonist approved for type 2 diabetes, can be repurposed to suppress colorectal cancer (CRC) by reprogramming tumor metabolism. Unlike conventional chemotherapies that aim to kill rapidly dividing cells, this research introduces a non-cytotoxic metabolic intervention, targeting the tumor's reliance on glucose metabolism—particularly glycolysis and the TCA cycle.
The study further reveals that TZP’s anti-cancer efficacy is independent of blood glucose levels, suggesting broad applicability not limited to diabetic patients. Its ability to reduce tumor burden across multiple CRC models, including orthotopic, subcutaneous, and patient-derived xenograft (PDX) models, underscores the drug’s robust and consistent in vivo performance. Notably, the research also demonstrates that TZP can synergize with standard chemotherapeutic agents, such as oxaliplatin, 5-fluorouracil (5-FU), and SN-38, potentially enhancing efficacy while reducing treatment toxicity.
These findings challenge the traditional dichotomy between metabolic diseases and cancer, proposing a translational strategy where metabolic drugs can serve as targeted anti-cancer agents, opening up new therapeutic avenues in precision oncology.
The Power of Spatial Metabolomics in Tumor Microenvironment Profiling
A particularly striking aspect of this research lies in its use of spatial metabolomics, a cutting-edge technique that enables high-resolution, region-specific metabolic profiling of tissue sections. By utilizing MALDI-MSI (Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging), the authors visualized how TZP altered glucose-derived metabolites exclusively in tumor regions, while sparing surrounding non-cancerous tissues. This level of spatial precision reveals tumor-specific metabolic vulnerabilities that bulk metabolomics would fail to detect.
Spatial metabolomics does more than provide metabolic snapshots—it enables researchers to decode the biochemical architecture of the tumor microenvironment. In this study, the technology offered critical insights into how HIF-1α-driven glycolysis was selectively downregulated in tumor zones, highlighting its value in mechanism-of-action studies, biomarker discovery, and target validation.
As cancer research increasingly focuses on tumor heterogeneity and microenvironmental dynamics, spatial metabolomics stands out as a transformative tool. Its applications extend beyond oncology into areas such as immunometabolism, drug resistance, and metabolic crosstalk, making it a foundational technology for next-generation translational research. With growing accessibility and analytical sophistication, spatial metabolomics is poised to play a central role in precision medicine and metabolic therapy development.
MetwareBio: Empowering Spatial Metabolomics for Translational Oncology
At MetwareBio, we are proud to empower transformative discoveries like this one through our state-of-the-art spatial metabolomics platform. Leveraging advanced MALDI-MSI technology and high-resolution timsTOF mass spectrometry, our services are designed to help researchers visualize, quantify, and interpret the spatial distribution of metabolites with unmatched clarity and precision. Our spatial metabolomics service enables you to:
- Map metabolic heterogeneity across different tissue regions (e.g., tumor core vs. peritumoral zones) to uncover localized metabolic reprogramming
- Achieve single-region or even sub-regional resolution, allowing for precise interpretation of the tumor microenvironment or drug effects
- Integrate with histology and transcriptomics data to build a multi-dimensional understanding of disease biology
- Detect hundreds of metabolites simultaneously, including those involved in glycolysis, TCA cycle, amino acid metabolism, nucleotide metabolism, and more
- Use results to guide target validation, mechanism-of-action studies, and biomarker discovery in cancer, immunology, neuroscience, and metabolic diseases
Our expertise spans from sample preparation to bioinformatics, ensuring a seamless and high-quality research experience. Whether your goal is to unravel drug mechanisms or discover biomarkers, MetwareBio’s mass spectrometry-based platforms and expert team are here to help. Contact us to get started!
Reference
Zhang, Y., Xie, Y., Xia, S., Ge, X., Li, J., Liu, F., Jia, F., Wang, S., Zhou, Q., Gao, M., Fang, W., & Zheng, C. (2025). The Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide Attenuates Colon Cancer Development by Regulating Glucose Metabolism. Adv. Sci. 2025, 2411980. https://doi.org/10.1002/advs.202411980
Read more
- Spatial Metabolomics: MALDI-MSI vs. AFADESI-MSI, Key Technologies, and Applications
- MALDI, DESI, or SIMS? How to Choose the Best MSI Techniques for Spatial Metabolomics
- Is Your Spatial Metabolomics Sample Ready?
- How to Prepare Samples for Spatial Metabolomics: The Essential Guide You Need
- Top FAQs on Spatial Metabolomics Sample Preparation
- Unlocking the Key Parameters of Spatial Metabolomics: Decoding Precision Metabolic Mapping
- Spatial Metabolomics Resolution Guide: From 100μm to 5μm in Plant Tissue Imaging
- Advanced Data Analysis in Spatial Metabolomics
- Spatial Metabolomics in Cancer Research: Unlocking the Metabolic Code for Precision Oncology
- Tracking Drugs in 4D: The Future of Pharmacokinetics with Spatial Metabolomics
- Spatial Metabolomics: Transforming Biomedical and Agricultural Research
- Unlocking Biological Complexity with Spatial Multi-Omics
- Spatial Multi-Omics Technologies: Unlocking the Spatiotemporal Code of Life


