Unlocking Biological Complexity with Spatial Multi-Omics
Living organisms are intricate systems where biological functions emerge not merely from individual cells or molecules but from the precise spatial organization and interactions of diverse cell types. Traditional omics technologies—such as transcriptomics, proteomics, and metabolomics—deliver rich molecular profiles but often homogenize tissue samples, stripping away critical spatial context. This "grinding-based" approach obscures tissue heterogeneity, cell-cell communication, and microenvironmental influences, limiting our ability to fully unravel complex biological processes.
From Single Cells to Spatial Context: The Rise of Spatial Multi-Omics
Single-cell omics revolutionized life sciences by decoding cellular heterogeneity through individual cell sequencing, revealing rare cell types and developmental trajectories. However, its reliance on dissociating cells disrupts native tissue architecture, sacrificing spatial information. Enter spatial multi-omics, a transformative suite of technologies that preserve spatial context while integrating molecular data across genes, proteins, and metabolites. By mapping molecular features directly within their native tissue environments, spatial multi-omics acts as a high-resolution lens, merging molecular biology with spatial topology. These tools complement single-cell omics: while the latter provides granular cell-type resolution, spatial multi-omics reconstructs their spatial relationships and interactions. Together, they forge multidimensional maps of life, accelerating discoveries in development, disease, and beyond.
Spatial Transcriptomics: Gene Expression Meets Tissue Architecture
Spatial transcriptomics emerged in 2013 with the introduction of ‘in situ’sequencing (FISSEQ), enabling high-throughput sequencing directly on intact tissue sections. Over the past decade, innovations like Visium (2016), Slide-seq (2018), and newer platforms have expanded its capabilities.
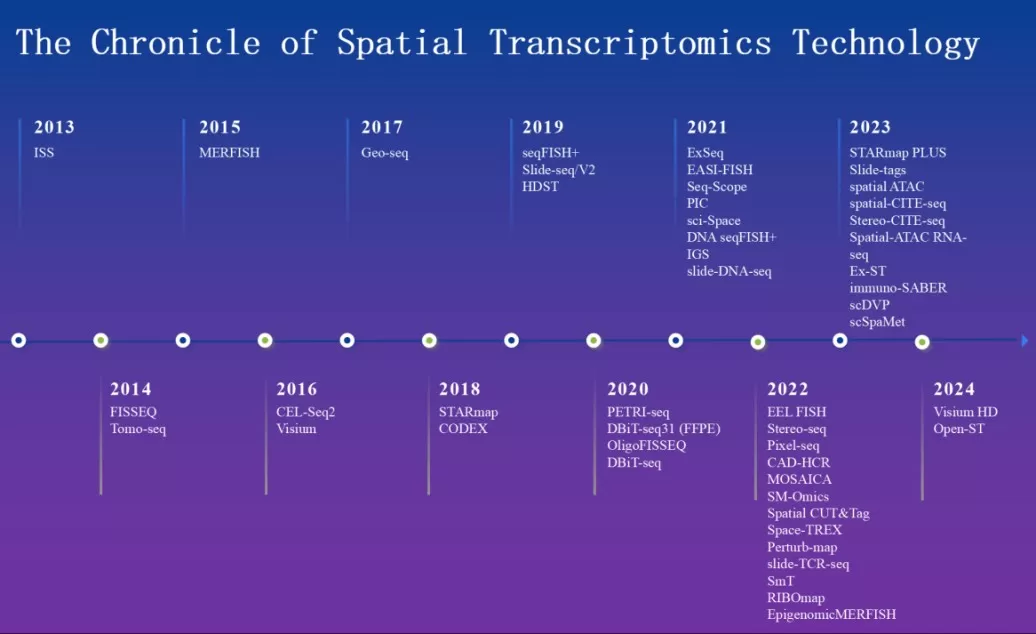
The Chronicle of Spatial Transcriptomics Technology
Four core methodologies dominate this field:
- Spatial ‘In Situ’ Hybridization (SISH): Fluorescent probes bind target mRNA, visualized via microscopy (e.g., seqFISH, MERFISH).
- Spatial ‘In Situ’ Sequencing (SISS): Combines hybridization with sequencing for direct spatial localization (e.g., FISSEQ, 10x Xenium).
- Spatial ‘In Situ’ Microdissection (SISM): Laser-based isolation of regions/cells for RNA-seq (e.g., Tomo-seq, DSP).
- Spatial ‘In Situ’ Barcoding (SISB): DNA barcodes tag mRNA for sequencing and spatial decoding (e.g., Slide-seq, DBiT-seq).
Recent advancements, such as subcellular-resolution platforms, are pushing boundaries in understanding tissue microenvironments and disease mechanisms.
Comparison of spatial transcriptomics technologies
|
Technology |
Examples |
Advantages |
Limitations |
|
SISH |
seqFISH, MERFISH |
High specificity; preserves spatial info |
Limited fluorescence channels; complex image analysis |
|
SISS |
FISSEQ, 10x Xenium |
Direct sequencing on tissue; retains spatial info |
Limited throughput; resolution varies by technology |
|
SISM |
Tomo-seq, DSP |
High resolution; compatible with various samples |
Low throughput; high cost |
|
SISB |
Slide-seq, DBiT-seq |
High resolution; long-read sequencing |
Resolution limits; high cost; complex data processing |
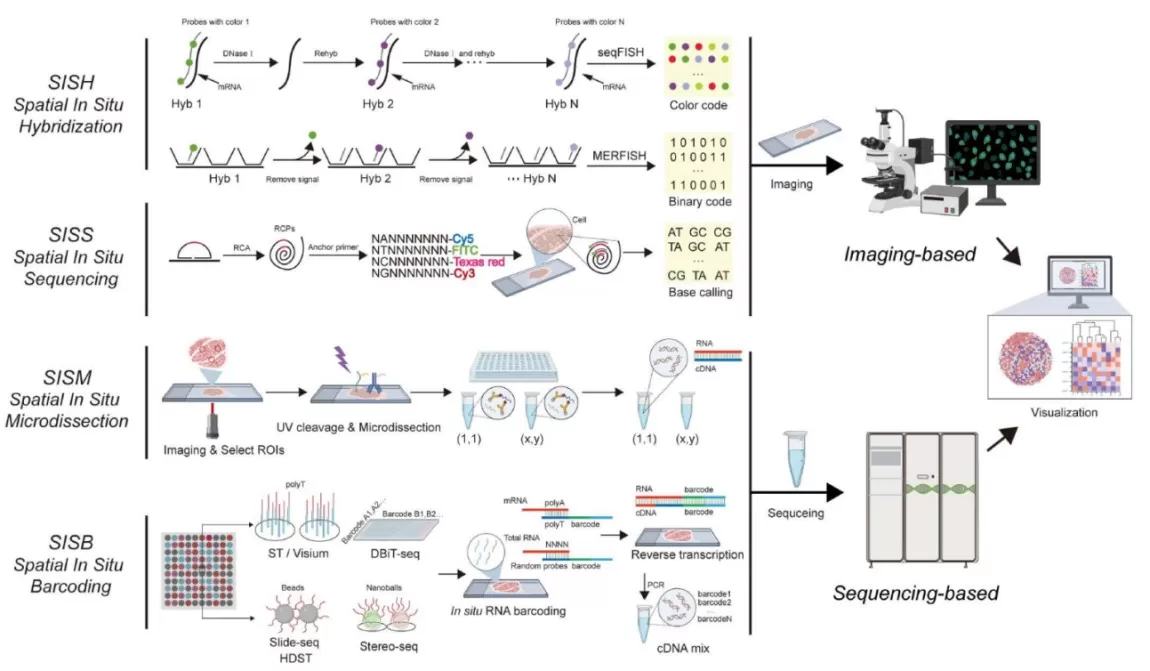
Four core methodologies of Spatial Transcriptomics
Spatial Proteomics: Visualizing Protein Networks in Context
Spatial proteomics bridges antibody-based imaging (e.g., cyclic immunofluorescence, CODEX) with cutting-edge mass spectrometry. In 2024, Nature Methods crowned Deep Visual Proteomics (DVP) as the "Method of the Year" for its ability to map proteomes without antibody limitations. DVP combines laser microdissection with high-sensitivity mass spectrometry, retaining spatial coordinates to generate precise protein distribution maps.
Key platforms include:
- Cyclic Immunofluorescence (cycIF): Iterative antibody staining for multiplexed protein detection.
- Imaging Mass Cytometry (IMC): Metal-tagged antibodies analyzed via time-of-flight mass spectrometry.
- Multiplexed Ion Beam Imaging (MIBI): Secondary ion mass spectrometry for ultrahigh-plex protein imaging.
These tools illuminate protein interactions in cancer microenvironments, immune responses, and neurological disorders, offering insights into disease progression and therapeutic targets.
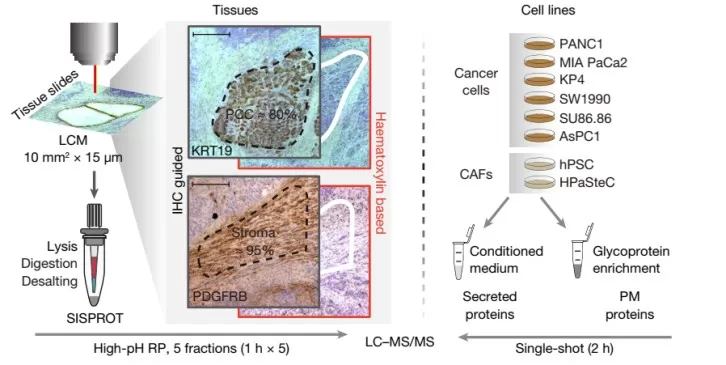
Functional proteomics of intercellular signalling in pancreatic cancer
Spatial Metabolomics: Decoding Small Molecules in 3D
Metabolomics, the closest omics layer to phenotype, gains spatial dimensionality through technologies like mass spectrometry imaging (MSI). By mapping metabolites in tissues, researchers pinpoint ‘where’ molecular changes occur, enhancing studies in cancer, neurodegeneration, and plant biology.
Core Techniques:
- MALDI-MSI: Matrix-assisted laser desorption/ionization for high-resolution (<1µm) metabolite
- DESI-MSI: Ambient ionization for rapid, non-destructive analysis.
- SIMS-MSI: High-resolution elemental and small-molecule imaging.
MALDI-MSI dominates life sciences due to its versatility, while DESI-MSI excels in clinical rapid diagnostics.
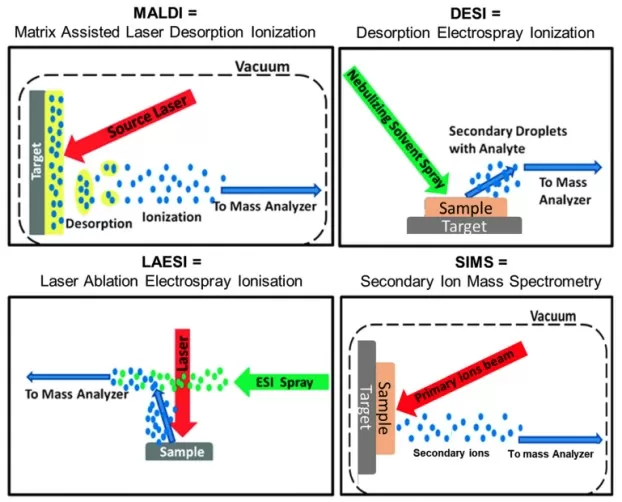
Simplified mechanistic diagrams for four most commonly used ionization techniques in imaging mass spectrometry
Translational Applications: Spatial Omics in Medicine and Biology
Spatial multi-omics is reshaping research across disciplines:
- Cancer Biology: Resolving tumor heterogeneity, metastasis, and drug resistance mechanisms.
- Developmental Biology: Mapping organogenesis and neural circuit formation.
- Precision Medicine: Identifying spatial biomarkers for personalized therapies.
- Plant Science: Studying stress responses and metabolic pathways in crops.
For example, combining spatial transcriptomics and proteomics has uncovered immune cell "hotspots" in tumors, guiding immunotherapy strategies. Similarly, MALDI-MSI has revealed metabolite gradients in Alzheimer’s brain tissues, linking spatial dysregulation to disease progression.
As spatial multi-omics evolves, integration with AI and multi-modal datasets will unlock predictive models of tissue behavior. Emerging technologies like ‘in situ’ genome sequencing and spatial epigenomics promise even deeper insights. With each innovation, we move closer to a holistic understanding of life’s spatial blueprint—transforming diagnostics, therapeutics, and beyond.
References:
Wang J, Ye F, Chai H, Jiang Y, Wang T, Ran X, Xia Q, Xu Z, Fu Y, Zhang G, Wu H, Guo G, Guo H, Ruan Y, Wang Y, Xing D, Xu X, Zhang Z. Advances and applications in single-cell and spatial genomics. Sci China Life Sci. 2024 Dec 20. doi: 10.1007/s11427-024-2770-x.
Huang P, Gao W, Fu C, Wang M, Li Y, Chu B, He A, Li Y, Deng X, Zhang Y, Kong Q, Yuan J, Wang H, Shi Y, Gao D, Qin R, Hunter T, Tian R. Clinical functional proteomics of intercellular signalling in pancreatic cancer. Nature. 2025 Jan;637(8046):726-735. doi: 10.1038/s41586-024-08225-y.
Dill AL, Eberlin LS, Ifa DR, Cooks RG. Perspectives in imaging using mass spectrometry. Chem Commun (Camb). 2011 Mar 14;47(10):2741-6. doi: 10.1039/c0cc03518a.


