Unlocking the Key Parameters of Spatial Metabolomics: Decoding Precision Metabolic Mapping
Spatial metabolomics, powered by mass spectrometry imaging (MSI), is revolutionizing the way we explore the spatial distribution of metabolites within biological tissues. Unlike traditional imaging techniques such as fluorescence imaging or radiolabeled tracing, MSI requires no chemical or radioactive labeling, preserves spatial metabolic information, and offers high sensitivity and specificity. As a result, it has gained significant traction in fields such as cancer research, biomarker discovery, drug development, and metabolic disease studies. To achieve optimal results in spatial metabolomics, several key factors must be carefully considered, including the choice of embedding media, matrix selection, and spatial resolution. This article delves into these critical parameters and their impact on spatial metabolomics analysis, providing insights into how researchers can optimize their workflow for precise and reproducible metabolic mapping.
MALDI MS Imaging: The Foundation of Spatial Metabolomics
Matrix assisted laser desorption/ionization (MALDI) mass spectrometry imaging serves as the cornerstone of spatial metabolomics. The technique involves coating tissue samples with a lightabsorbing matrix, which crystallizes with metabolites. When irradiated by a UV laser (337 nm or 355 nm), the matrix transfers energy to the metabolites, ionizing them for detection. A precise XYstage moves the sample systematically, allowing the mass spectrometer to collect spatially resolved data points. These data are then reconstructed into highresolution images, revealing metabolite distributions across the tissue. MALDIMS imaging covers a broad mass range (50–1300 Da), making it ideal for detecting endogenous metabolites, exogenous drugs, and their derivatives.
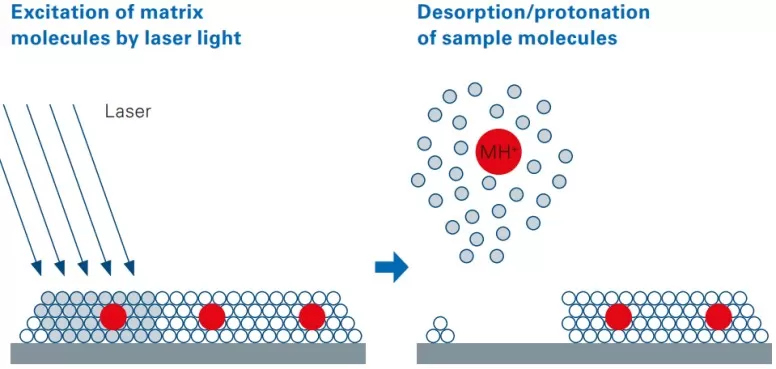
The mechanism of MALDI
The Spatial Metabolomics Workflow: From Sample to Insight
The spatial metabolomics workflow consists of multiple crucial steps, each of which directly impacts the accuracy and reliability of the results. The key steps in a typical spatial metabolomics experiment include:
1. Sample Collection – The collection of high-quality biological tissue samples under optimal conditions to preserve metabolic integrity.
2. Embedding and Sectioning – The tissue is embedded in a medium to facilitate cryosectioning. Thin tissue sections (typically 5-50 μm) are obtained using a cryostat to maintain spatial integrity.
3. Matrix Application – A chemical matrix is uniformly applied to the tissue surface to assist in ionization during mass spectrometry analysis.
4. Mass Spectrometry Imaging – The tissue section is analyzed using MALDI-MSI, where a laser systematically scans the sample, generating a mass spectrum for each spatial location.
5. Data Processing and Analysis – Specialized computational tools are used to process the acquired data, reconstruct spatial maps of metabolite distribution, and perform statistical analysis to derive meaningful biological insights.
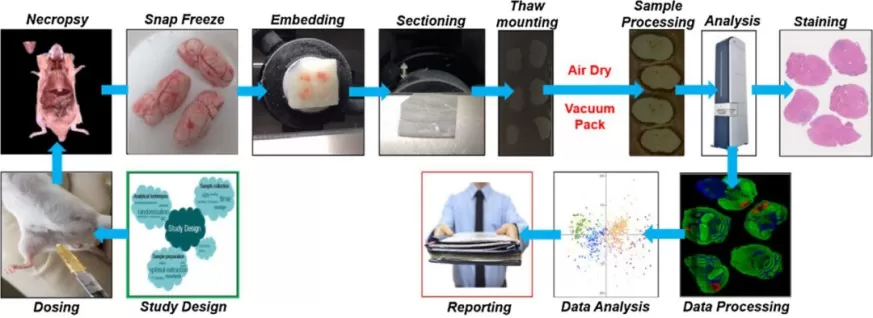
The workflow of spatial metabolomics
Among these steps, embedding media selection, matrix optimization, and resolution settings profoundly influence data quality. Below, we delve into these critical parameters.
Embedding Media: Guardians of Spatial Integrity
Embedding media serve as the unsung guardians of spatial metabolomics, playing a crucial role in preserving tissue morphology, stabilizing metabolites, facilitating sectioning, and minimizing background noise. The selection of the appropriate embedding medium directly influences the accuracy of metabolic profiling.
Commonly Used Embedding Media:
- Carboxymethyl cellulose (CMC): A widely used medium due to its minimal interference with metabolite detection. It remains liquid at room temperature but solidifies upon freezing, making it ideal for cryosectioning of biological samples such as animal and plant tissues. It provides good adhesion between tissue and slides, reducing detachment risks during sectioning and analysis.
- FSC22 Blue Embedding Medium: Designed specifically for cryosectioning, this medium ensures optimal tissue hardness at low temperatures, making sectioning easier while maintaining metabolic integrity. It is particularly beneficial for soft tissues, ensuring smoother sectioning and reduced mechanical stress.
- Optimal Cutting Temperature (OCT) Compound: Frequently used in pathology, OCT offers excellent support for sectioning but introduces background interference that can hinder metabolite detection. While useful for histological analysis, it is less suitable for spatial metabolomics due to its impact on metabolite stability.
- Gelatin: Although it causes little metabolic interference, it has limitations due to its tendency to adhere to tissue sections, complicating sample handling. It is useful in specific cases where an alternative to synthetic polymers is required.
- Paraffin: Typically used for histological analysis rather than metabolomics due to its significant impact on metabolic integrity. Paraffin embedding requires dehydration and solvent treatment, which can degrade metabolites, making it unsuitable for MSI applications.
Among these, CMC and FSC22 stand out as the most suitable choices for spatial metabolomics applications due to their minimal metabolic interference and excellent sectioning properties. Optimizing embedding media selection is a crucial first step toward ensuring reliable and high-resolution metabolomic data.
Matrix Spraying: The Catalyst for Ionization Efficiency
The application of the matrix in MALDI-MS imaging is a crucial step that significantly affects ionization efficiency, signal stability, and spatial resolution. A properly selected matrix enhances ionization, protects metabolites from degradation, and reduces background noise.
Commonly Used Matrices and Their Applications
- 2,5-Dihydroxybenzoic acid (DHB): Versatile and widely used, DHB is effective for detecting lipids, carbohydrates, and small metabolites in positive ion mode. It forms homogeneous crystals, making it suitable for broader sample types but with moderate spatial resolution.
- α-Cyano-4-hydroxycinnamic acid (CHCA): Offers high sensitivity for amino acids, peptides, organic acids, and nucleotides, making it particularly valuable for high-resolution imaging. CHCA tends to form finer crystals, leading to improved spatial resolution compared to DHB.
- 9-Aminoacridine (9-AA): Ideal for negative ion mode, 9-AA enhances the detection of acidic metabolites such as organic acids, nucleotides, and certain carbohydrates. It provides excellent sensitivity for acidic compounds, often yielding better contrast in metabolic imaging.
The choice of matrix, along with optimized application techniques, significantly impacts metabolite ionization and detection efficiency. Spray coating and sublimation are commonly used methods to ensure uniform matrix application, ultimately leading to better spatial resolution and reproducibility.
Resolution: The Key to Spatial Heterogeneity
In MSI, spatial resolution refers to the smallest area from which ions are detected and analyzed. This parameter directly influences the granularity of the metabolic map and determines the level of biological detail that can be captured. Higher resolution improves spatial detail but increases acquisition time and data complexity. Researchers must balance resolution needs with computational feasibility.
For example, in the imaging of rapeseed tissue (diameter ~2mm), comparative analyses have shown that metabolic features become more distinct at 20 μm resolution, with further improvements at 10 μm and 5 μm, particularly for structural differentiation at tissue boundaries. However, the trade-off between resolution and acquisition time must be considered, as higher resolution results in increased data acquisition time and larger datasets to process.
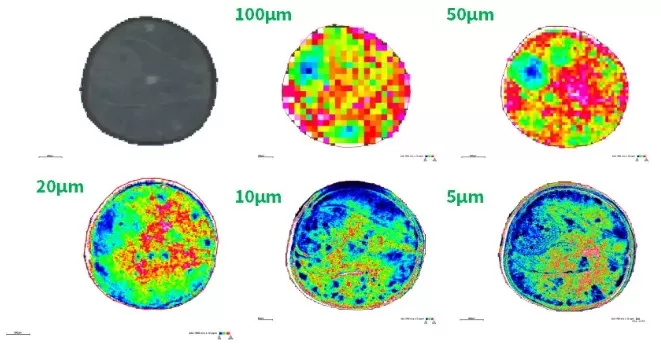
The spatial distribution map of one molecule in rapeseed at different spatial resolution
Guidelines for Resolution Selection:
- Larger tissues (e.g., entire organ sections) may be adequately analyzed at 50-100 μm resolution.
- Tissues with fine microstructures (e.g., kidney tubules, brain tissue) require higher resolutions, typically 5-20 μm.
- For high-precision studies, a resolution of 5 μm is optimal, enabling detailed metabolic heterogeneity analysis at the cellular level.
Conclusion: Toward Precision Metabolic Imaging
Spatial metabolomics integrates cutting-edge mass spectrometry imaging techniques to reveal the spatial distribution of metabolites within biological samples, providing invaluable insights for biomedical research. The precision of these analyses hinges on three key parameters:
1. Embedding Media: Selecting the right medium (e.g., CMC, FSC22) ensures tissue preservation and metabolic stability.
2. Matrix Application: Choosing an appropriate matrix (e.g., DHB, CHCA, 9-AA) enhances ionization efficiency and signal stability.
3. Spatial Resolution: Adjusting resolution (e.g., 5-100 μm) optimizes the level of detail captured in metabolic mapping.
By optimizing these parameters, spatial metabolomics continues to push the boundaries of precision medicine, drug discovery, and systems biology. As technology advances, innovations in sample preparation, data acquisition, and analytical techniques will further refine the accuracy and applicability of spatial metabolomics, unlocking new frontiers in metabolic research.
At MetwareBio, we are committed to providing comprehensive spatial metabolomics solutions, from sample preparation to data analysis, ensuring high-quality and reproducible results. Contact us today to learn how our expertise can support your research needs!


