Spatial Metabolomics Explained: How It Works and Its Role in Cancer Research
Introduction
Spatial metabolomics is a cutting-edge field that provides a deeper understanding of cancer biology by mapping the distribution of metabolites within tissues. Unlike traditional metabolomics, which provides a global overview of metabolism, spatial metabolomics incorporates the dimension of space, allowing researchers to study the distribution of metabolites in different regions of a tissue. Due to the complex metabolic reprogramming and different microenvironments exhibited by tumors, spatial metabolomics has great promise in early cancer detection, treatment development, and personalized medicine.
Understanding Metabolomics
Metabolomics is the comprehensive study of metabolites—the small molecules involved in metabolism, such as amino acids, lipids, sugars, and other biochemical compounds. These metabolites serve as end-products of cellular processes and provide a real-time snapshot of an organism's metabolic state. By analyzing the full complement of metabolites, metabolomics offers a powerful tool to study physiological conditions, disease states, and therapeutic responses.
In cancer, metabolomics can provide critical insights into the altered metabolic pathways that tumors rely on to grow and survive. Unlike genomics or proteomics, which focus on genetic or protein data, metabolomics directly reflects the biochemical processes occurring within cells, making it highly relevant for studying diseases like cancer.
How Spatial Metabolomics Works and the Technologies Used
Spatial metabolomics takes the principles of traditional metabolomics a step further by adding a spatial dimension to the analysis. It involves analyzing metabolites within their native tissue environments, mapping how they are distributed across different regions. This enables researchers to understand the metabolic heterogeneity within tissues, which is particularly important in cancer, where different regions of a tumor may display varying metabolic activities.
There are three main technologies used in spatial metabolomics:
-
Mass Spectrometry Imaging (MSI):
MSI is one of the most widely used technologies in spatial metabolomics. It combines the power of mass spectrometry with imaging to create detailed spatial maps of metabolite distribution in tissue sections. MSI allows for the identification and visualization of metabolites at a high spatial resolution, making it invaluable for studying tumors and their microenvironments. -
Laser Capture Microdissection (LCM):
LCM is another technique that enables precise sampling of specific tissue regions. It allows researchers to isolate individual cells or areas of interest from a tissue sample, which can then be analyzed for their metabolic content. This is particularly useful for studying the tumor microenvironment, where distinct regions (e.g., tumor core vs. periphery) may exhibit different metabolic profiles. -
Secondary Ion Mass Spectrometry (SIMS) and MALDI Imaging:
Emerging techniques like SIMS and Matrix-Assisted Laser Desorption/Ionization (MALDI) imaging are also making their way into spatial metabolomics. These methods offer high resolution and sensitivity, allowing researchers to capture finer details of metabolite distribution within tissues.
The process typically begins with careful tissue preparation. Tissue samples are often frozen or fixed to preserve metabolic activity. Once prepared, they un
Applications of Spatial Metabolomics in Cancer Research
Spatial metabolomics has a profound impact on cancer research, offering insights that traditional methods cannot provide. Its applications in cancer research are diverse and include:
-
Tumor Microenvironment Profiling:
Tumors are not just made up of cancer cells. They also contain various non-cancerous cells, including immune cells, fibroblasts, and blood vessels, which form the tumor microenvironment (TME). Spatial metabolomics enables researchers to study the metabolic interactions within the TME, shedding light on how cancer cells communicate with their surrounding environment to promote growth and resist treatments. -
Understanding Cancer Metabolism:
Cancer cells exhibit altered metabolism to support rapid growth and survival, a phenomenon known as the Warburg effect. Spatial metabolomics allows researchers to map how these metabolic changes occur within tumors, revealing areas of high glycolytic activity or oxidative metabolism. This insight is essential for developing targeted therapies that can disrupt these metabolic processes. -
Identifying Biomarkers for Early Detection:
By studying the metabolic profiles of different regions within a tumor, spatial metabolomics can help identify biomarkers associated with cancer. These biomarkers could potentially be used for early cancer detection, offering a non-invasive way to diagnose cancer at its earliest stages, even before the onset of symptoms.
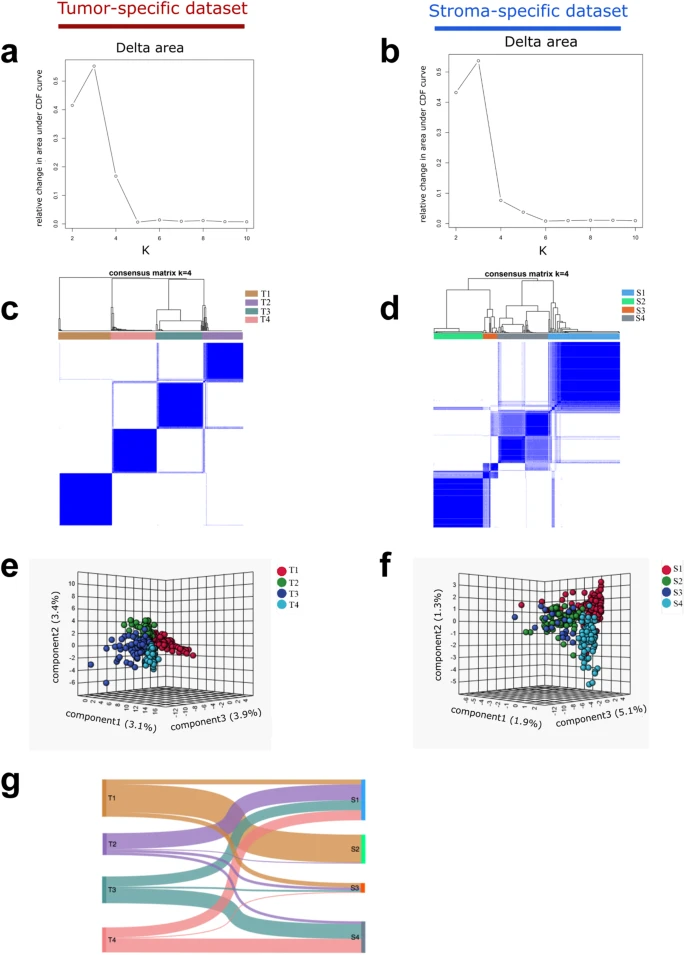
Identification of tumor- and stroma-specific subtypes and their association with molecular features (wang et al. 2023)
As spatial metabolomics continues to evolve, several exciting developments are on the horizon:
-
Integration with Other Omics Technologies:
One of the most promising future directions is the integration of spatial metabolomics with other omics technologies, such as genomics, proteomics, and transcriptomics. By combining data from multiple omics layers, researchers can gain a more comprehensive understanding of cancer biology, improving diagnostic accuracy and therapeutic targeting. -
Technological Advancements:
The resolution and sensitivity of spatial metabolomics technologies continue to improve. As new imaging techniques and mass spectrometry methods emerge, the ability to detect and map metabolites with even greater precision will open up new possibilities for studying tumor biology. -
Clinical Applications and Personalized Medicine:
The ultimate goal of spatial metabolomics is to make it a routine tool in clinical oncology. By analyzing the metabolic profiles of tumors, spatial metabolomics can guide personalized treatment strategies, identifying which therapies are most likely to be effective for individual patients based on their unique metabolic signatures.
Personalized medicine aims to tailor treatments based on individual patient characteristics, and spatial metabolomics plays a pivotal role in this process. By mapping the metabolic signatures of tumors, spatial metabolomics can provide essential information on how a patient's tumor might respond to different therapies. This approach enables more targeted treatments, reducing side effects and improving patient outcomes.
Spatial metabolomics also aids in the development of novel therapeutic agents. By identifying key metabolic pathways that are altered in cancer, researchers can discover new drug targets and design therapies that specifically disrupt these pathways, making treatments more effective and less toxic.
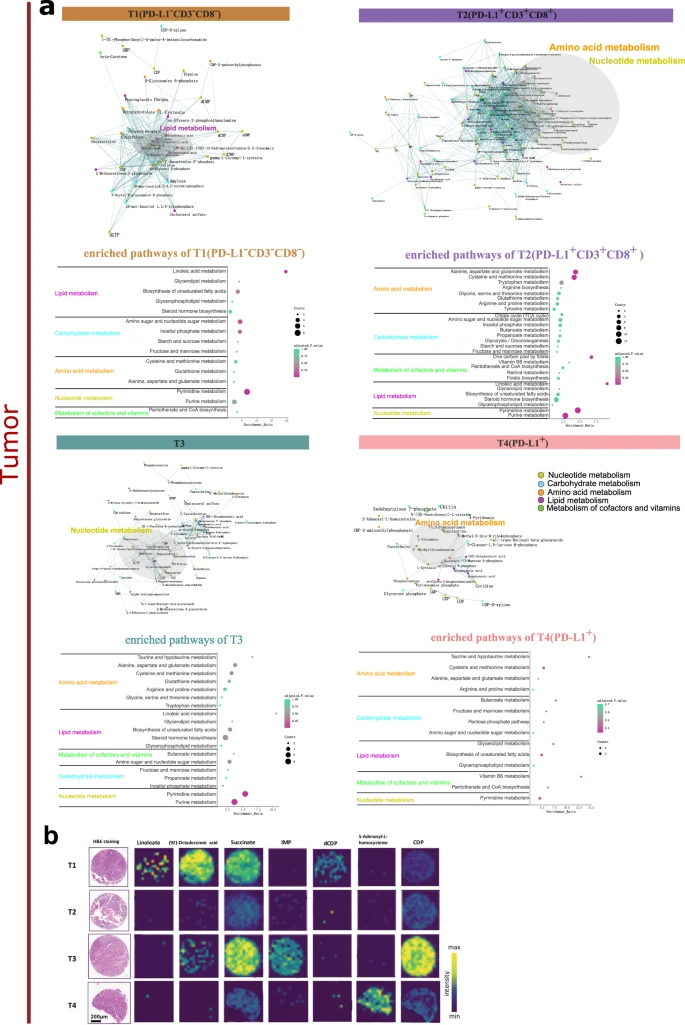
Metabolite characteristics and enriched pathways of tumor subtypes (Wang et al. 2023)
In the battle against cancer, spatial metabolomics is a powerful ally. It offers us a map—a map of the metabolic landscape that reveals how tumors thrive, how they spread, and how they resist treatment. This knowledge will guide us to earlier detection, more personalized therapies, and ultimately, better outcomes for cancer patients.
As spatial metabolomics continues to evolve, it will play an increasingly central role in clinical practice, offering a window into the very heart of the disease. The future of cancer research is bright, and with each discovery, we move closer to unlocking the mysteries of cancer and, one day, conquering it.
If you are looking for reliable metabolomics services, contact MetwareBio immediately. We will provide you with the most convenient and efficient testing solutions.
FAQ
What is spatial metabolomics?
-
Spatial metabolomics is the study of metabolites within their natural tissue context, mapping their distribution and understanding how their locations influence biological processes, particularly in diseases like cancer.
How does spatial metabolomics help cancer research?
-
It provides insights into the metabolic changes occurring within tumors, helping researchers understand how cancer cells adapt, grow, and resist treatment. It also aids in identifying biomarkers for early detection.
What technologies are used in spatial metabolomics?
-
Mass spectrometry imaging (MSI) and laser capture microdissection (LCM) are key technologies that allow for the mapping and analysis of metabolites within tissues.
Can spatial metabolomics be applied to other diseases?
-
Yes, spatial metabolomics holds promise for studying other diseases, including neurological disorders and cardiovascular diseases, by providing detailed insights into their metabolic landscapes.
How will spatial metabolomics impact personalized medicine?
-
Spatial metabolomics will enable doctors to tailor cancer treatments based on the unique metabolic signatures of each patient’s tumor, leading to more effective and targeted therapies.
Reference
Wang, J., Sun, N., Kunzke, T. et al. Spatial metabolomics identifies distinct tumor-specific and stroma-specific subtypes in patients with lung squamous cell carcinoma. npj Precis. Onc. 7, 114 (2023). https://doi.org/10.1038/s41698-023-00434-4
Read more
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


