Unlocking Precision in Spatial Metabolomics: Essential Detection Parameters for Cutting-Edge Research
Introduction to Spatial Metabolomics and Its Advantages
Spatial metabolomics, an emerging molecular imaging technology, is rooted in mass spectrometry imaging (MSI). Unlike traditional metabolomics, which often loses spatial information during homogenization, spatial metabolomics enables qualitative, quantitative, and localized analysis of metabolites within intact biological tissues. By preserving the spatial architecture of samples, this technique provides a macroscopic view of metabolic activity across organs or tissue regions, uncovering dynamic molecular interactions in their native context. For instance, in cancer research, spatial metabolomics can map metabolite gradients around tumors, revealing how metabolic dysregulation propagates through adjacent tissues. This capability addresses a critical limitation of conventional approaches, where spatial heterogeneity—key to understanding disease mechanisms—is often averaged out. The integration of spatial and molecular data opens new avenues for studying drug distribution, biomarker discovery, and metabolic pathways in situ.
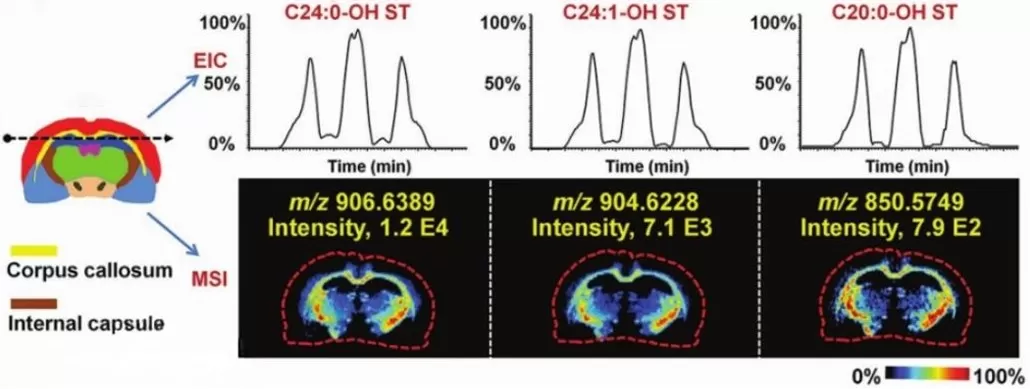
Spatial metabolomics metabolite assays
Detection Techniques in Spatial Metabolomics
The mainstream spatial metabolomics platforms include MALDI-MSI (Matrix-Assisted Laser Desorption/Ionization), DESI-MSI (Desorption Electrospray Ionization), and AFADESI-MSI (Air Flow-Assisted Desorption Electrospray Ionization). Among these, MALDI-MSI stands out for its high spatial resolution (down to 5×5 μm), making it ideal for small or intricate samples like neuronal tissues or biopsy sections. MALDI also covers a broad molecular weight range (100–1300 Da), enabling detection of diverse metabolites, lipids, and exogenous compounds.
How MALDI-MSI Works:
- A sample is coated with a UV-absorbing matrix (e.g., DHB or CHCA) on a conductive plate.
- A pulsed laser (337 nm nitrogen or 355 nm Nd:YAG) irradiates the matrix, triggering desorption and ionization of analytes.
- Ionized molecules are accelerated into a mass analyzer for ‘m/z’ separation and detection.
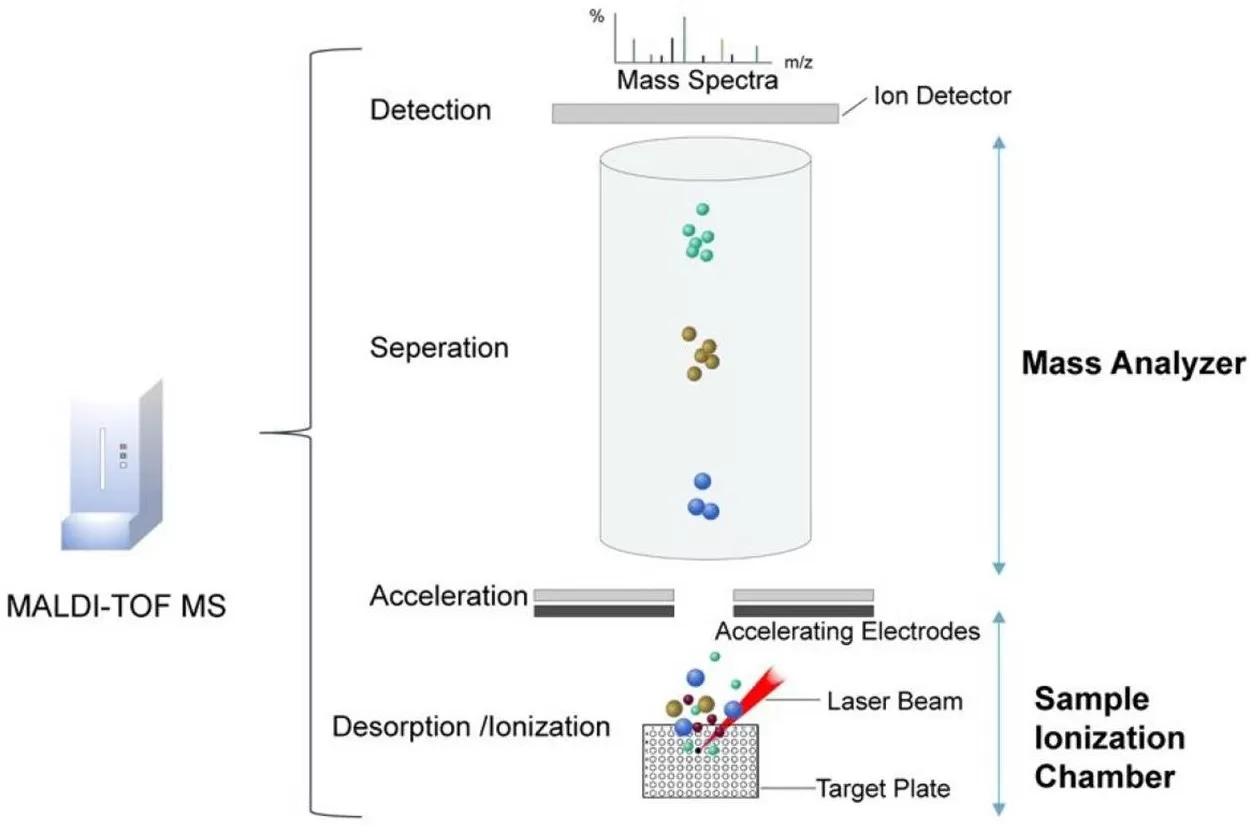
MALDI-TOF MS analysis for spatial metabolomics
In contrast, DESI and AFADESI operate under ambient conditions without matrix application, favoring rapid profiling of larger tissue areas. However, their spatial resolution (~50–200 μm) is lower than MALDI, limiting fine-grained analysis.
The Role of Matrix Selection in MALDI-MSI
The matrix is pivotal in MALDI-MSI, acting as a mediator for energy transfer and analyte ionization. Common matrices include:
- 2,5-Dihydroxybenzoic acid (DHB): Ideal for carbohydrates and small molecules.
- α-Cyano-4-hydroxycinnamic acid (CHCA): Optimized for peptides and lipids.
- 9-Aminoacridine (9-AA): Suitable for negative-ion mode analysis of metabolites like nucleotides.
Matrix choice directly impacts sensitivity and ionization efficiency. For example, DHB forms fine crystals that enhance signal uniformity, while CHCA improves detection of hydrophobic compounds. Improper matrix selection can lead to ion suppression or crystallization artifacts, underscoring the need for method optimization based on analyte properties.
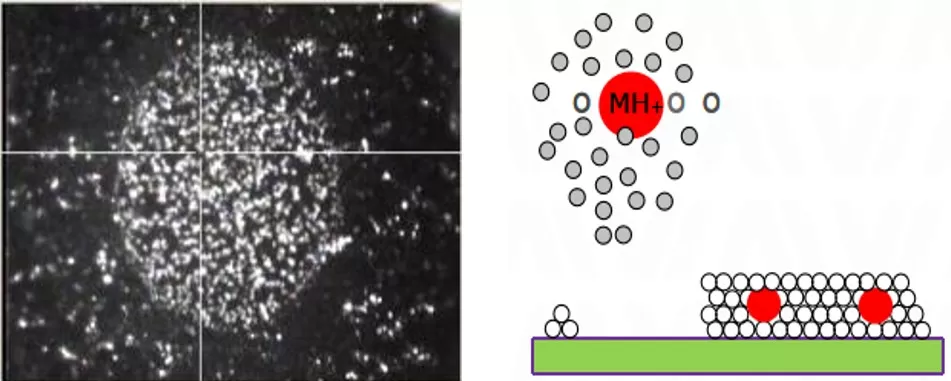
Spatial metabolomics matrix
Key Parameters: Spatial and Mass Resolution
Spatial metabolomics relies on two resolution metrics: spatial resolution and mass resolution. Spatial Resolution is defined as the smallest distinguishable distance between two points on a sample, spatial resolution determines imaging granularity. MALDI-MSI achieves micron-level precision by focusing laser beams to discrete spots. Higher resolution (e.g., 10 μm vs. 50 μm) enables detailed mapping of microstructures, such as tumor margins or neuronal synapses. However, ultra-high resolution increases acquisition time and data complexity, necessitating a balance between detail and practicality. Mass resolution reflects a mass spectrometer’s ability to distinguish ions with similar ‘m/z’ values (e.g., isotopes or structural isomers). High-resolution instruments are critical for identifying low-abundance metabolites in complex mixtures. For example, distinguishing glucose (m/z 179.056) from its isomer fructose requires resolution exceeding 50,000.
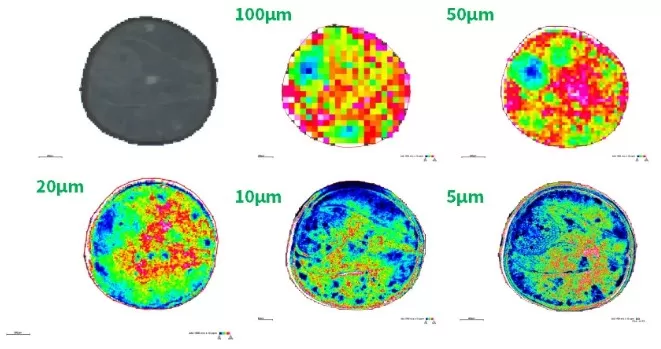
Spatial metabolomics resolution
Spatial metabolomics is poised to revolutionize precision medicine and systems biology. By unraveling the spatial dynamics of metabolism, this technology will deepen our understanding of organ function, drug efficacy, and pathological processes, ultimately guiding personalized therapeutic strategies.
References
1. Li D, Yi J, Han G, Qiao L. MALDI-TOF Mass Spectrometry in Clinical Analysis and Research. ACS Meas Sci Au. 2022 Jul 27;2(5):385-404. doi: 10.1021/acsmeasuresciau.2c00019.
2. Buchberger AR, DeLaney K, Johnson J, Li L. Mass Spectrometry Imaging: A Review of Emerging Advancements and Future Insights. Anal Chem. 2018 Jan 2;90(1):240-265. doi: 10.1021/acs.analchem.7b04733.
Read more
Spatial Metabolomics: Transforming Biomedical and Agricultural Research
Spatial Metabolomics Explained: How It Works and Its Role in Cancer Research
MALDI, DESI, or SIMS? How to Choose the Best MSI Techniques for Spatial Metabolomics
How to Prepare Samples for Spatial Metabolomics: The Essential Guide You Need


