Spatial Metabolomics Based on Mass Spectrometry
1. Introduction
Metabolites are the products of chemical reactions in cells and organisms, and they play a key role in maintaining life. Studying the composition, quantity and function of metabolites is an important way to understand the physiological and pathological state of organisms, which is the core task of metabolomics. Traditional metabolomics methods usually provide insights into the global metabolic state by collecting the overall metabolic data of samples for analysis. However, the limitation of this method is that it cannot reveal the spatial distribution characteristics of metabolites, especially the inability to analyze the metabolic differences in different tissues and cell regions.
Spatial metabolomics breaks through this limitation and provides us with more detailed and efficient metabolic research tools by analyzing the spatial distribution of metabolites at the tissue section and single cell level. Unlike traditional metabolomics, spatial metabolomics can accurately locate metabolites in different locations of tissues and reveal the spatiotemporal changes of metabolic activities by combining mass spectrometry technology.
2. Spatial Metabolomics Methods
Spatial metabolomics based on mass spectrometry can analyze metabolites from submicron to meter level or larger, and can also combine mass spectrometry data with spatial information of tissues through imaging mass spectrometry (IMS), allowing researchers to obtain spatial distribution maps of metabolites in tissues. This method can not only provide high-resolution metabolite data, but also reveal the metabolic characteristics of different cell populations by analyzing metabolite changes in different tissue regions. The most common methods include matrix-assisted laser desorption/ionization (MALDI) mass spectrometry imaging (MSI), secondary ion mass spectrometry (SIMS), and desorption electrospray ionization (DESI) MSI. Another emerging technology is single-probe MSI, which can be used to perform single-cell and tissue analysis.
Sample selection and preparation
Sample selection and preparation are crucial in spatial metabolomics. Appropriate samples not only ensure the integrity of metabolites, but also improve the accuracy of analytical results. Common sample preparation methods include frozen sections, fixation, and dehydration processes. During sample preparation, it is necessary to avoid the degradation of metabolites and ensure that the physical state of the sample does not interfere with the distribution of metabolites during analysis.
In all cases, careful and appropriate sample preparation methods are crucial. They should limit metabolite degradation, maintain metabolite sample distribution (e.g., by avoiding diffusion or delocalization of metabolites), and preserve overall tissue biogeography or integrate sufficient reference points to correct for sample deformation introduced, for example, during tissue section drying. Choices such as fixation, matrix selection, desorption solvent, metabolite extraction solvent, data acquisition instrument, and data acquisition polarity will determine the metabolite classes that can be detected in a given spatial metabolomics experiment. Although a broad metabolite coverage is usually the goal, complete metabolite coverage is currently not possible, and typically only a small fraction of metabolites in a given sample can be detected and detected. The metabolite classes that can be detected will depend on the sample preparation and mass spectrometry technology selected.
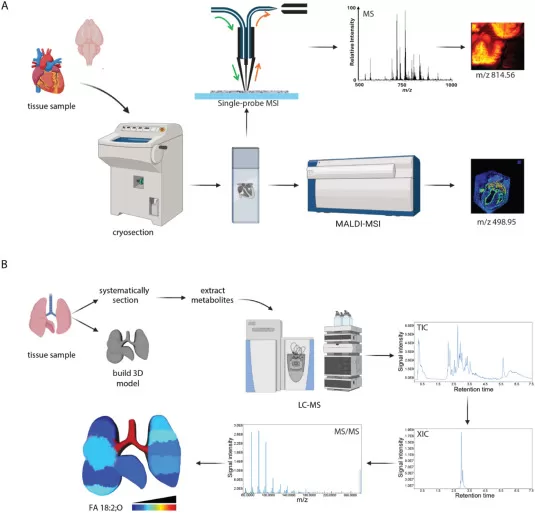
Representative spatial metabolomics workflows (Wheeler et al., 2024)
3. Representative Applications in Health
Spatial metabolomics can provide considerable insight into the role of location in healthy organ function as well as disease processes.
3.1. Understanding the brain
Given its unique structure, the brain is often used as a biological system to validate and illustrate the benefits of novel spatial metabolomics techniques. For example, the original publication on 3D OrbiSIMS used the technique to reveal fine spatial distribution of cholesterol and phosphoinositides in the mouse hippocampus, accumulation of adenine at the single-nucleus level, and accumulation of the γ-aminobutyric acid neurotransmitter in the granular layer region. Similarly, water GCIB-SIMS showed differences in the abundance of specific sulfates, phosphatidylinositols, glutathione, adenosine monophosphate, and fatty acids between the outer, middle, granular layers, and white matter of the mouse cerebellar cortex.
Spatial metabolomics techniques can also provide functional insights into brain physiology. Specifically, major challenges in our understanding of the brain include mapping the relationship between chemical signaling, brain anatomy, and brain function, and distinguishing between changes associated with healthy aging and pathological alterations. For example, tissue sections, metabolite extraction, and liquid chromatography or gas chromatography (GC)-MS are used to compare metabolites in specific regions of the mouse brain of different ages and sexes. The results showed that age-dependent effects were larger for both sexes, including different patterns of sphingolipid species abundance, providing a chemical mechanism for myelin degeneration. Furthermore, functionally similar regions showed similar metabolism, providing an important link between chemistry and physiology.
3.2. Understanding the microbiota
The mammalian microbiome, the community of microorganisms that live in and on mammals, is restricted to specific sites of colonization. However, studies in germ-free and antibiotic-treated mice have revealed widespread effects of the microbiome on organs distal to the site of microbiome colonization. Spatial metabolomics approaches can be used to identify metabolite mediators and metabolic consequences of these widespread effects. For example, a systematic “chemical mapping” comparison of tissue metabolite levels in different organ segments in germ-free and colonized mice identified novel bile acid conjugates produced by the microbiota: phenylacetic acid, tyrosocholic acid, and leucocholic acid. These metabolites were primarily found in the ileum, duodenum, and jejunum of colonized mice, whereas detection of the same conjugated bile acids was decreased 10-fold in the cecum and colon.
However, the integration of metabolomics analysis with microbiome data can be challenging. Limitations of current dissection methods result in a loss of spatial resolution at the level of individual cells, and the bacterial component of the microbiome is equal to or below the spatial resolution of MALDI-MSI. SIMS studies of microorganisms have either focused on elemental analysis or on environmental or pathogenic microorganisms. In addition, the identity of many microbiome metabolites remains unknown. Indeed, this is a particular challenge in metabolomics studies, as a considerable number of signals cannot currently be annotated, either because they are instrumental artifacts or because they represent novel chemicals (particularly those derived from microorganisms). Advances in genomic and metabolite databases and new data processing tools will help address the latter issue. One emerging approach is dual MALDI-MSI and fluorescence in situ hybridization (FISH) to identify specific microorganisms that locally produce target metabolites. Improved communication between microbiome researchers and bioanalytical chemists focused on small molecule characterization will enable further expansion of spatial metabolomics techniques to study the microbiome.
4. Representative Applications in Disease
4.1. Cancer
Spatiality plays a crucial role in cancer pathophysiology and treatment, influencing key aspects such as tumor margin definition and the study of metastasis sites and their consequences. One of the significant clinical applications of spatial metabolomics is the determination of tumor boundaries during surgery, helping surgeons make informed decisions in real-time. Moreover, spatial metabolomics has provided valuable insights into cancer pathogenesis by offering a detailed understanding of the tumor microenvironment (TME).
For instance, research utilizing MALDI-MSI (Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging) has uncovered significant metabolic patterns within the TME. Studies have revealed high levels of acylcarnitines at the tumor periphery, showing an inverse correlation with ATP levels. This suggests that acylcarnitines may be involved in metabolic reprogramming at the edges of the tumor, possibly influencing tumor progression.
Similarly, research has shown that ovarian tissue produces norepinephrine when exposed to transformed cells derived from the fallopian tube, and this neurotransmitter enhances the invasiveness of cancer cells. These findings highlight how tumor-associated cells can modify local metabolism, contributing to malignancy progression.
Further advancements in spatial metabolomics have been achieved through multi-modal data acquisition techniques. For example, airflow-assisted DESI-MSI (Desorption Electrospray Ionization Mass Spectrometry Imaging) was employed to measure increased proline levels in esophageal squamous cell carcinoma. By integrating this approach with immunohistochemistry, researchers found that the rate-limiting enzyme in proline biosynthesis was also upregulated in these tumors, revealing a direct connection between metabolic shifts and tumor pathology. This simultaneous analysis of metabolites and key enzymes in glutamine metabolism demonstrated consistent patterns that may serve as novel biomarkers for cancer diagnosis and prognosis.
In another breakthrough, SIMS (Secondary Ion Mass Spectrometry) was used to analyze both protein and metabolite distributions in the breast cancer tumor microenvironment. Lanthanide-labeled antibodies allowed researchers to pinpoint specific biomarkers, revealing higher levels of lysophosphatidic acid (LPA 20:0) in rapidly proliferating cells (identified by Ki-67-positive regions). The study also identified increased polyunsaturated lipids in infiltrating macrophages, suggesting a link between lipid metabolism and immune cell infiltration in tumors.
Expanding on these methodologies to analyze additional protein markers and cancer types holds great promise. The integration of spatial proteomics and metabolomics could revolutionize cancer therapy, enabling the development of more targeted and personalized treatment strategies.
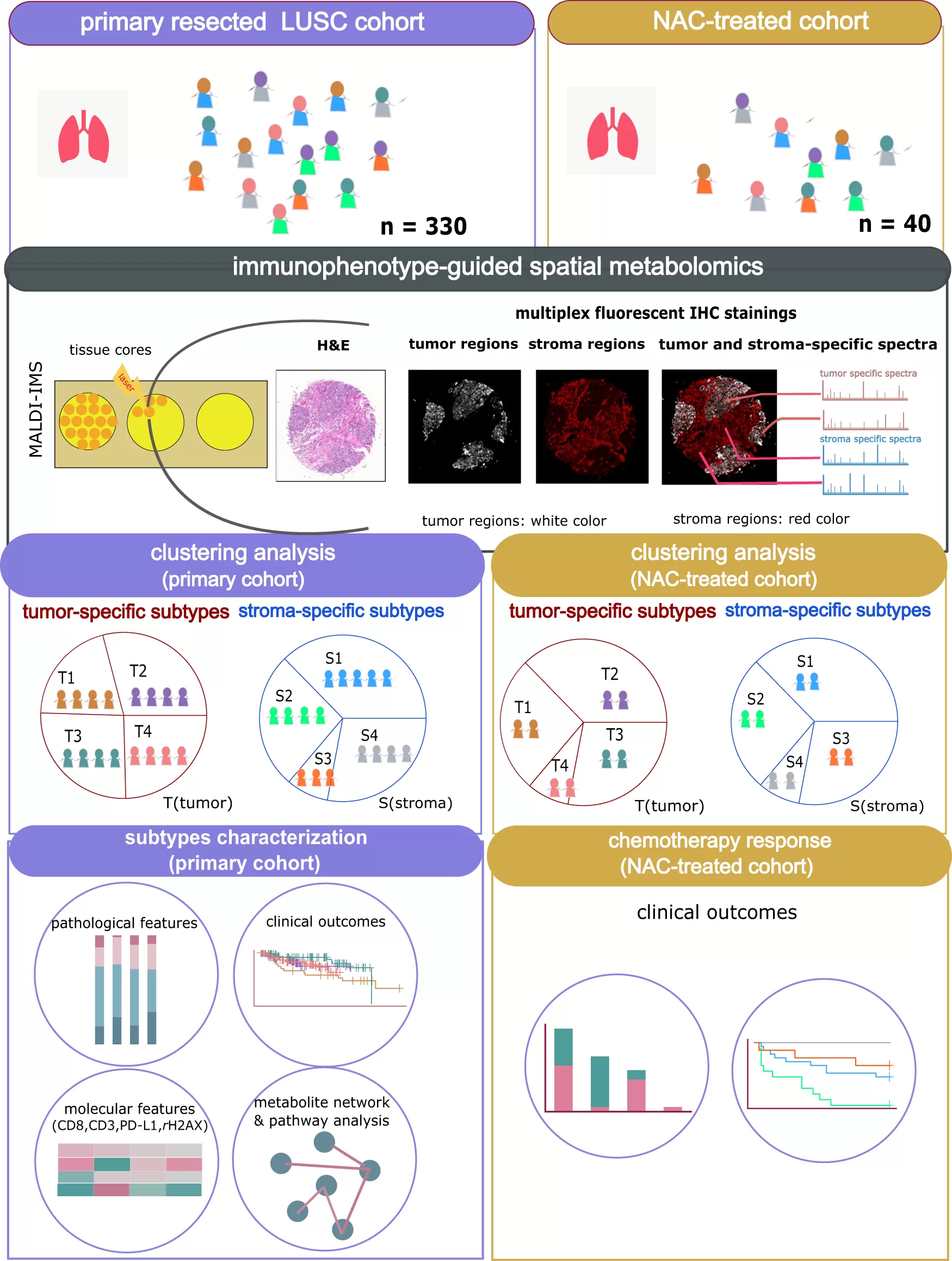
(Wang et al., 2023)
4.2. Neurological disorders
Spatial metabolomics plays a crucial role in understanding brain function and its alterations in neurological disorders, including neurodegenerative diseases, traumatic brain injury, and stroke. Recent studies have used advanced mass spectrometry imaging techniques, such as MALDI-MSI, to reveal distinct metabolic changes in various brain regions associated with these conditions.
In the context of Parkinson’s disease, spatial metabolomics has been used to analyze brain regions affected by the disease. Studies employing MALDI-MSI in rodent models have shown that damaged areas in the brain exhibit a decrease in critical neurotransmitters such as dopamine and norepinephrine. Furthermore, treatments with levodopa, a common therapeutic drug for Parkinson’s disease, were found to elevate γ-aminobutyric acid (GABA) levels in the striatum, which plays a key role in motor control and cognition. Similarly, in Alzheimer’s disease models, MALDI-MSI identified reduced levels of gangliosides in the cerebellum and the right hemisphere of mice. These findings may provide deeper insights into the pathophysiology of Alzheimer’s, helping to uncover potential biomarkers for early diagnosis and treatment response.
Stroke research has also benefitted from spatial metabolomics, with studies using MALDI-MSI to highlight significant metabolic differences between infarcted and healthy regions of the brain. For instance, post-stroke brain analysis in animal models revealed elevated levels of glucose and citric acid, while key metabolites such as guanosine monophosphate (GMP), adenosine diphosphate (ADP), inosine, and glutathione were notably reduced in infarcted areas. These alterations are thought to reflect metabolic shifts due to restricted oxygen availability and the inability of the affected brain regions to combat oxidative stress. Furthermore, these studies demonstrated how spatial metabolomics can assess the effects of potential therapies aimed at reversing such metabolic abnormalities, offering a pathway for evaluating treatment efficacy in stroke recovery.
In traumatic brain injury (TBI) research, spatial metabolomics has proven essential in characterizing metabolic changes following injury. Using GCIB-SIMS, researchers have observed significant alterations in lipid profiles in brain regions such as the ipsilateral contusional cortex and the hippocampal CA3 region in rats. The CA3 region is critical for memory, and the observed loss of polyunsaturated cardiolipin in this area may help explain cognitive deficits following brain injury.
Moreover, in TBI studies, specific oxidized lipids such as phosphatidylethanolamine PE (38:4)-OH and arachidonic acid AA (20:4)-OH were found to accumulate in the affected brain areas, while non-oxidized phosphatidylethanolamine levels decreased. These findings suggest that lipid peroxidation is a critical factor in TBI-related damage. Additional 3D MALDI-MSI analyses further confirmed the increase in phosphatidylcholine (PC(42:9)) at lesion sites, which could serve as a biomarker for TBI severity.
Importantly, spatial metabolomics can also help assess the effectiveness of therapeutic interventions. For instance, in TBI models, MALDI-MSI was used to track the impact of a "decoy" peptide treatment, revealing a reduction in ceramide levels at the injury site. This demonstrates how spatial metabolomics not only aids in understanding the metabolic underpinnings of neurological injuries but also facilitates the evaluation of new therapeutic strategies aimed at improving brain recovery.
5. Frontiers in Spatial Metabolomics Research
With the continuous advancement of spatial metabolomics technology, related fields are also developing rapidly. The following are some of the current cutting-edge research directions in this field, revealing the possible future development trends of spatial metabolomics.
Spatial metabolomics is benefiting from the rapid development of mass spectrometry technology, and new mass spectrometers and analytical methods are constantly emerging. Among them, the combination of three-dimensional mass spectrometry imaging and single-cell mass spectrometry technology has brought higher spatial resolution to spatial metabolomics. This method can accurately capture the distribution of metabolites in the three-dimensional space of tissue sections, allowing researchers to deeply explore the spatial heterogeneity of metabolic processes. As the technology continues to mature, future mass spectrometry technology will help to further improve the accuracy and reliability of metabolomics research.
Improving the confidence of data annotation is a key technical challenge in spatial metabolomics. With the improvement of algorithms and data processing methods, researchers are now able to more accurately calibrate and quantify metabolites in spatial data. Higher annotation confidence not only enhances the accuracy of metabolite identification, but also provides more reliable quantitative information for research. This progress enables spatial metabolomics research to more accurately reveal the relationship between metabolic networks and biological functions, and improve the reproducibility and reliability of experimental results.
The integration of spatial metabolomics with other omics (such as transcriptomics, proteomics, etc.) is becoming an important trend in future research. By combining spatial metabolomics with data such as transcriptomics and proteomics, researchers can obtain more comprehensive metabolic and functional information of biological systems. This multi-omics data fusion helps to better understand the complex metabolic processes, molecular interactions and their functions in biological systems. In addition, the analysis methods combining different omics data can deeply reveal the relationship between metabolites, genes and proteins, and provide a more comprehensive perspective for disease mechanisms and treatment strategies.
As the complexity of spatial metabolomics data increases, new visualization technologies are being developed to help researchers intuitively present metabolite distribution. The application of three-dimensional visualization technology and virtual reality (VR) technology enables spatial metabolomics data to be presented in a more vivid and easier to understand way. Researchers can use virtual reality technology to observe the distribution of metabolites in tissues in real time, thereby more clearly showing the metabolic differences between different regions. These innovative visualization methods not only improve the efficiency of data analysis, but also help provide more influential results in academic exchanges and scientific research presentations.
Explore Advanced Spatial Metabolomics With MetwareBio
Overall, including a spatial aspect to metabolomics analyses grants a new level of understanding of the role of metabolism in disease pathogenesis, lending itself to more effective diagnostic, prevention, and treatment methods in a spatial precision medicine framework.
As a leading provider of spatial metabolomics and proteomics services, MetwareBio is at the forefront of biotechnology advancements, using comprehensive analytical techniques to enhance the understanding and application of human proteins. Our state-of-the-art services are designed to support industry in tackling the complexity of metabolomics, lipidomics and proteomics, empowering your business with precise, innovative solutions. Visit us at www.metwarebio.com to learn how MetwareBio can transform your product development.
Reference
1. K. Wheeler et al., Frontiers in mass spectrometry-based spatial metabolomics: Current applications and challenges in the context of biomedical research, TrAC Trends in Analytical Chemistry, Volume 175, 2024, 117713, ISSN 0165-9936, https://doi.org/10.1016/j.trac.2024.117713.
2. Wang, J., Sun, N., Kunzke, T. et al. Spatial metabolomics identifies distinct tumor-specific and stroma-specific subtypes in patients with lung squamous cell carcinoma. npj Precis. Onc. 7, 114 (2023). https://doi.org/10.1038/s41698-023-00434-4
Read More
MALDI, DESI, or SIMS? How to Choose the Best MSI Techniques for Spatial Metabolomics


