SDS-PAGE: A Cornerstone of Protein Analysis in Modern Biochemistry
In the fields of biochemistry and molecular biology, protein research is pivotal for unraveling life processes and disease mechanisms. Among the array of analytical techniques, SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) stands out as a gold standard for protein separation. Renowned for its high resolution, reproducibility, and versatility, this method has become indispensable in laboratories worldwide. This blog delves into the principles, workflow, and critical applications of SDS-PAGE, while exploring its evolving role in cutting-edge research.
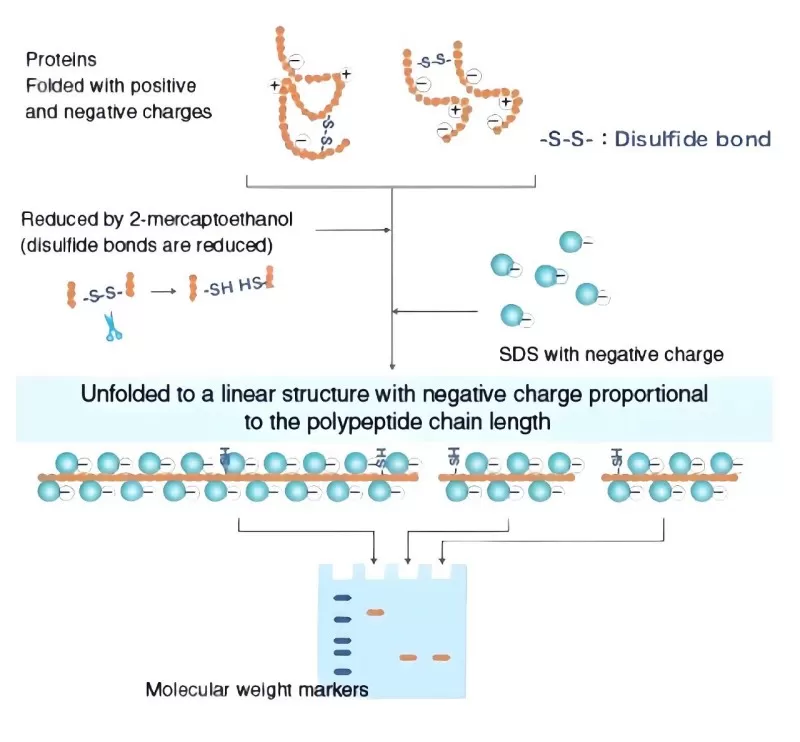
SDS-PAGE is an analytical technique that separates proteins based on their molecular weight. By combining the denaturing power of sodium dodecyl sulfate (SDS) with the sieving effect of polyacrylamide gel electrophoresis (PAGE), this method resolves complex protein mixtures with exceptional precision. The polyacrylamide gel matrix is formed through the polymerization of acrylamide (Acr) and the crosslinker N,N'-methylenebisacrylamide (Bis), catalyzed by ammonium persulfate (APS) and N,N,N',N'-tetramethylethylenediamine (TEMED). This creates a three-dimensional mesh that acts as a molecular sieve during electrophoresis.
Core Principles of SDS-PAGE
1) Charge Uniformity: The Role of SDS
SDS, an anionic detergent, binds to proteins at a ratio of approximately 1.4g SDS per 1g of protein. This interaction disrupts non-covalent bonds (e.g., hydrogen, hydrophobic, and ionic bonds), denaturing proteins and conferring a uniform negative charge. Consequently, intrinsic charge differences among proteins are masked, ensuring migration during electrophoresis depends solely on molecular weight. Reducing agents like dithiothreitol (DTT) or β-mercaptoethanol are often added to break disulfide bonds, linearizing multi-subunit proteins and eliminating shape-related migration artifacts.
2) Molecular Sieving: The Gel Matrix
The pore size of the polyacrylamide gel is determined by the concentrations of acrylamide and Bis. For example: - 8–15% gels: Ideal for separating most proteins (10–250 kDa).
Gradient gels: Resolve proteins with subtle molecular weight differences (e.g., 5–20% gradients).
Under an electric field, smaller proteins navigate the gel pores more rapidly, while larger ones lag, enabling size-based separation.
3) The Discontinuous Buffer System
SDS-PAGE employs a two-layer buffer system to enhance resolution:
- Stacking gel (pH 6.8): Low acrylamide concentration and large pores compress proteins into narrow bands using glycine’s zwitterionic state.
- Separating gel (pH 8.8): Higher acrylamide concentration and smaller pores separate proteins by molecular weight as glycine becomes fully negatively charged, accelerating migration.
Schematic representation of SDS-PAGE workflow
Key Applications of SDS-PAGE
1) Molecular Weight Determination
By comparing protein migration distances to a ladder of known molecular weights (e.g., Precision Plus Protein™ Standards), researchers estimate the size of unknown proteins. This is critical for initial characterization in studies involving novel proteins or engineered variants.
2) Assessing Purity and Homogeneity
A single, sharp band on a gel indicates a pure sample, while multiple or smeared bands suggest impurities or degradation. This is invaluable for optimizing purification protocols—for instance, monitoring column chromatography eluates to eliminate contaminants.
3) Subunit Composition Analysis
Multi-subunit proteins (e.g., antibodies or enzyme complexes) are dissociated into individual subunits under reducing conditions. SDS-PAGE reveals subunit molecular weights, aiding structural studies. For example, analyzing hemoglobin subunits helps diagnose thalassemia variants.
4) Protein Expression and Post-Translational Modifications
Combined with Western blotting or mass spectrometry, SDS-PAGE enables:
- Expression profiling: Comparing protein levels across conditions (e.g., tumor vs. normal tissues).
- Post-translational modification (PTM) analysis: Detecting phosphorylation or glycosylation shifts, which alter migration patterns.
SDS-PAGE is an irreplaceable tool in protein science, offering insights into molecular weight, purity, structure, and function. Its synergy with modern techniques like CRISPR screening and single-cell proteomics ensures its relevance in decoding complex biological systems. As research evolves, SDS-PAGE will undoubtedly remain a cornerstone of biochemical discovery, bridging classical methods with next-generation innovations.
Read more
Top-Down vs. Bottom-Up Proteomics: Unraveling the Secrets of Protein Analysis
Comprehensive Guide to Basic Bioinformatics Analysis in Proteomics
Strategies for Selecting Key Interacting Proteins in IP-MS Studies


