Protein Succinylation: A Key Post-Translational Modification
Protein succinylation is a significant post-translational modification, primarily occurring on lysine residues. This modification involves the covalent attachment of a negatively charged four-carbon succinyl group (-CO-CH2-CH2-CO2H) to the ε-amino group of lysine via succinyl-CoA. Succinylation can occur through two mechanisms: enzymatic and non-enzymatic. In the enzymatic process, specific succinyltransferases facilitate the transfer, while the non-enzymatic pathway involves the direct transfer of the succinyl group from succinyl-CoA. This modification plays a crucial role in regulating protein structure, stability, and function, and is involved in a range of biological processes, including cellular metabolism, signal transduction, and gene expression regulation.
Chemical Process of Succinylation
Succinylation is a post-translational modification involving the transfer of a succinyl group to the lysine residues of substrate proteins. This process can occur through either enzymatic or non-enzymatic mechanisms.
Enzymatic Succinylation
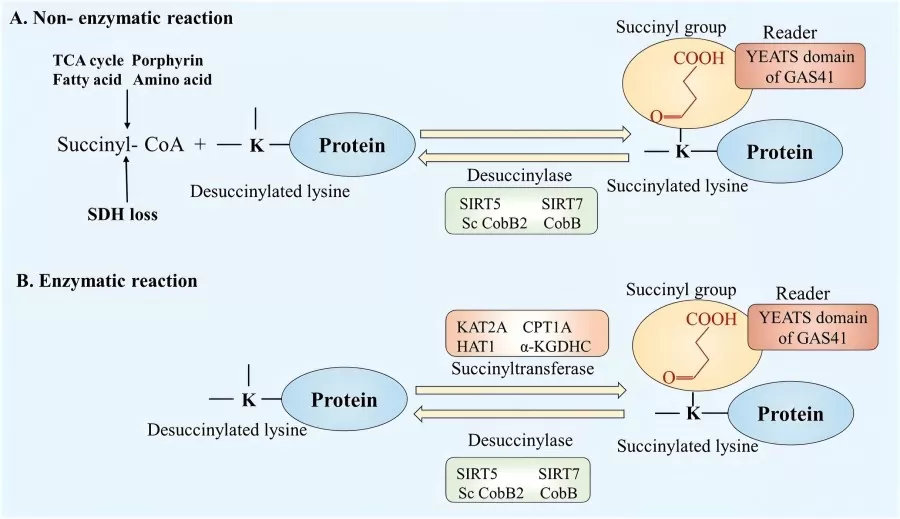
In the enzymatic process of protein succinylation, specific enzymes, such as succinyltransferases, catalyze the transfer of a succinyl group to lysine residues on target proteins. The process begins with the enzyme recognizing and binding to specific lysine residues, which are typically exposed on the protein surface, making them accessible for modification. In the enzyme's active site, succinic acid or succinyl-CoA acts as the donor of the succinyl group (-SCO2H). The succinyl group is then covalently attached to the ε-amino group of the lysine residue. This step involves the formation of a new covalent bond between the succinyl group and the lysine, releasing coenzyme A (CoA) or succinic acid as byproducts. Once the succinylation is complete, the modified protein is released from the enzyme to perform its biological functions.
Several types of enzymes are involved in this process, with Succinyl-CoA N6-acetyltransferase (SCoAT) being one of the most well-characterized, catalyzing the transfer of succinyl groups from succinyl-CoA to lysine residues. However, the full range of enzymes involved in succinylation is not yet fully understood, and other enzymes may contribute to this process.
The regulation of these enzymes can occur through additional post-translational modifications such as phosphorylation or acetylation. Furthermore, the intracellular concentrations of metabolites like succinic acid and succinyl-CoA play a critical role in modulating enzyme activity and the extent of succinylation.
Non-enzymatic succinylation
Non-enzymatic succinylation occurs through direct chemical reactions without the involvement of specific enzymes. In this process, succinic acid or its derivatives can react directly with lysine residues in proteins to form succinylated lysine. This reaction may require specific conditions, such as particular pH levels, temperatures, or redox states. Additionally, in certain circumstances, free radical-mediated reactions can also lead to protein succinylation. For instance, free radicals generated under oxidative stress conditions may promote succinylation.
Non-enzymatic reactions tend to be less specific than enzymatic ones, often occurring on various lysine residues, which can result in unpredictable succinylation patterns. Furthermore, because these reactions are not regulated by enzymes, they can be more random and uncontrollable in their occurrence.
Effects of Protein Succinylation
As a post-translational modification, protein succinylation alters the properties of proteins by adding a succinyl group (a side chain composed of two carboxyl groups and a methyl group) to the lysine residues. This modification can have wide-ranging effects on protein structure, function, and intracellular signaling.
Modulation of Protein Structure
Succinylation can induce changes in the local or overall conformation of a protein by adding a larger side chain to lysine residues. These conformational changes may impact the active site of the protein, thereby influencing its function. For instance, some enzymes have active sites that include lysine residues, and succinylation may regulate catalytic efficiency by altering the charge and spatial configuration of these residues.
The stability of a protein is closely related to its correctly folded state. Succinylation can affect protein folding and stability by altering the internal charge distribution and hydrophobic interactions within the protein. Chaperone proteins assist in the proper folding of other proteins, and succinylation may influence the interactions between these chaperones and target proteins, impacting the folding and aggregation states of the proteins.
Impact on Enzyme Activity and Interactions
Succinylation can directly or indirectly influence enzyme activity. The direct effect occurs through changes in the structure of the active site, while the indirect effect involves modifications to enzyme stability or interactions with other molecules. For example, succinylation may affect the activity of key enzymes in glycolysis, such as pyruvate kinase, thereby regulating energy metabolism.
The functionality of proteins largely depends on their interactions with other proteins or molecules. Succinylation can promote or inhibit these interactions by altering the surface characteristics of proteins. For instance, certain transcription factors regulate gene expression by interacting with specific DNA sequences or co-activators. Succinylation may modify these interactions, thus affecting gene expression. Additionally, succinylation can signal for protein degradation via the ubiquitin-proteasome pathway. For example, certain cyclins are ubiquitinated and degraded at specific phases of the cell cycle, and succinylation may influence the ubiquitination process of these proteins, thereby regulating the cell cycle.
Cellular Functions of Succinylation
Cell Metabolism Regulation
Succinylation plays a crucial role in regulating cellular metabolism, particularly in key metabolic pathways such as glycolysis and the tricarboxylic acid (TCA) cycle. By modulating the activity of critical enzymes within these pathways, succinylation can significantly influence metabolic flux. For instance, it may alter the activity of phosphofructokinase-1 (PFK-1) in glycolysis, thereby regulating the rate and direction of glucose metabolism. In the TCA cycle, succinylation can affect the functions of key enzymes such as citrate synthase and isocitrate dehydrogenase, ultimately influencing energy production and the generation of metabolic intermediates. This regulatory mechanism allows cells to adapt their metabolic state flexibly in response to environmental changes and energy demands.
Gene Expression Regulation
In addition to metabolic regulation, succinylation plays an important role in gene expression control. At the transcriptional level, it can affect the binding affinity of transcription factors to DNA, thereby regulating the expression of specific genes. Furthermore, succinylation may influence the activity of RNA polymerase, impacting transcription efficiency. During translation, it can modify initiation factors or ribosomal proteins, affecting the initiation and elongation phases of protein synthesis. These regulatory mechanisms work together to ensure that cells can accurately express the necessary proteins under specific conditions.
Cell Signaling
Succinylation also significantly impacts cellular signaling pathways. For example, in the PI3K/Akt signaling pathway, succinylation may modulate the activity of Akt kinase, influencing cell survival, growth, and metabolism. In the MAPK pathway, it can affect the phosphorylation state and activity of MAP kinases, thereby regulating cell differentiation, proliferation, and stress responses. Through these signaling pathways, succinylation plays a critical role in how cells respond to external signals.
Detection Techniques for Protein Succinylation
Immunoprecipitation and Western Blotting
To detect succinylated lysine residues, specific antibodies against succinylated lysines must be generated. This process involves immunizing animals, extracting the antibodies, and purifying them through affinity chromatography. These antibodies can selectively recognize and bind to succinylated proteins. The antibodies are mixed with cell extracts, allowing them to bind to the target proteins. The resulting antibody-protein complexes are then captured using immunomagnetic beads. Following this, the precipitated proteins are separated by electrophoresis, transferred to a membrane, and detected using specific antibodies.
Affinity Enrichment Coupled with Mass Spectrometry
Affinity enrichment materials are selected and prepared, typically consisting of affinity resins based on succinic acid or its derivatives, which specifically capture succinylated proteins or peptides. The proteins or peptides are mixed with the affinity materials to enrich for succinylated peptides. Bound peptides are then eluted using an appropriate elution buffer and purified. Finally, the purified succinylated peptides are analyzed using mass spectrometry.
Zhao, G., Zhen, J., Liu, X., Guo, J., Li, D., Xie, J., & Xie, L. (2022). Protein post-translational modification by lysine succinylation: Biochemistry, biological implications, and therapeutic opportunities. Genes & diseases, 10(4), 1242–1262. https://doi.org/10.1016/j.gendis.2022.03.009
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


