Comparative Guide to Protein Separation and Detection Chromatography: RPLC vs. HILIC vs. IEX vs. GPC/SEC vs. AC
Protein separation and characterization remain critical challenges in bioanalytical research due to the structural complexity and functional diversity of proteins. Liquid chromatography (LC) techniques have become indispensable tools for resolving these challenges, offering high resolution, flexibility, and scalability. This article provides an in-depth comparison of five major chromatography methods—Reversed-Phase Liquid Chromatography (RPLC), Hydrophilic Interaction Liquid Chromatography (HILIC), Ion-Exchange Chromatography (IEX), Gel Permeation/Size-Exclusion Chromatography (GPC/SEC), and Affinity Chromatography (AC)—highlighting their principles, applications, and emerging trends.
1. Reversed-Phase Chromatography (RPC)
1.1 Principle of RPC
RPC separates proteins or peptides based on hydrophobicity differences. The stationary phase consists of hydrophobic materials (e.g., C18-bonded silica), while the mobile phase is a gradient of water and organic solvents (e.g., acetonitrile/methanol). Analytes are eluted in order of polarity, with more hydrophobic molecules having longer retention times.
1.2 Protein Separation Based on Hydrophobicity
The core principle of RPC is hydrophobic interaction between the stationary phase (typically C18 chains) and the mobile phase (polar aqueous solutions). More hydrophobic molecules interact more strongly with the stationary phase, resulting in longer retention times. The separation process involves:
- Stationary and mobile phases: The stationary phase is usually hydrophobic (e.g., C18 or C8 alkyl chains bonded to silica), while the mobile phase is a polar solvent (e.g., water with acetonitrile or methanol) often supplemented with acid (e.g., formic acid) to enhance ion-pairing.
- Separation mechanism: Molecules interact with the stationary phase based on hydrophobicity. More hydrophobic molecules have longer retention times. Hydrophobicity is determined by amino acid composition, with certain residues (e.g., tryptophan, phenylalanine) being more hydrophobic.
- Elution methods: Gradient elution: Increasing the organic solvent concentration reduces mobile phase polarity, eluting molecules with different hydrophobicities. Isocratic elution: A constant mobile phase composition is used for molecules with small hydrophobicity differences.
1.3 Hydrophobicity and Separation
- Stationary phase characteristics: Hydrophobic alkyl chains (e.g., C18) bonded to silica particles. Larger pores improve diffusion for large molecules, while higher surface coverage provides more interaction sites.
- Hydrophobicity differences: Determined by amino acid composition. Hydrophobic residues (e.g., tryptophan, phenylalanine) increase retention time.
- Gradient elution: Increasing organic solvent concentration reduces mobile phase polarity, eluting more hydrophobic molecules.
- Ion-pair chromatography: Acids in the mobile phase (e.g., formic acid) form ion pairs with charged groups, enhancing interaction with the stationary phase.
- Molecular size and diffusion: Larger molecules have lower diffusion coefficients and migrate more slowly. Secondary structures (e.g., α-helices) also influence interactions.
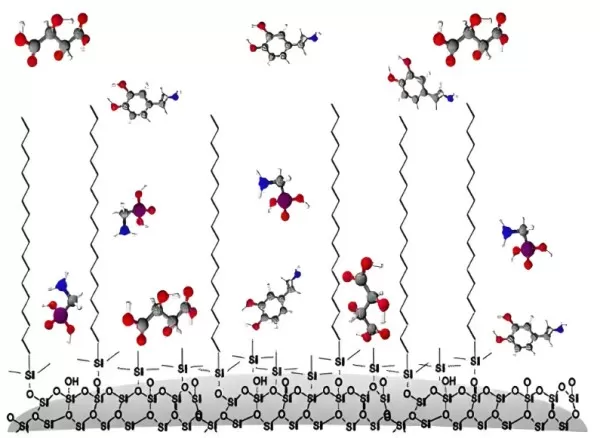
Packing for reversed-phase preparative chromatography
2. Hydrophilic Interaction Liquid Chromatography (HILIC)
2.1 Principle of HILIC
HILIC separates proteins or peptides based on hydrophilicity differences. The stationary phase consists of polar materials (e.g., silica or amino columns), while the mobile phase is a high proportion of organic solvent (e.g., acetonitrile) with a small amount of water. More hydrophilic molecules interact more strongly with the stationary phase, resulting in longer retention times.
2.2 Protein Separation Based on Hydrophilicity
The core principle of HILIC is hydrophilic interaction, utilizing a hydrophilic stationary phase and a relatively hydrophobic mobile phase to separate polar compounds. The separation process involves:
- Stationary and mobile phases: The stationary phase is typically polar (e.g., amino, diol, or silica), while the mobile phase is a high proportion of organic solvent (e.g., acetonitrile) with water.
- Sample loading: The sample is dissolved in a polar solvent and injected into the column.
- Separation mechanism: Molecules interact with the stationary phase based on hydrophilicity. More polar molecules have longer retention times. Hydrophilicity is determined by chemical properties, with certain residues (e.g., serine, threonine) being more hydrophilic.
- Elution methods: Gradient elution: Increasing water content enhances mobile phase polarity, eluting molecules with different hydrophilicities. Isocratic elution: A constant mobile phase composition is used for molecules with small hydrophilicity differences.
2.3 Hydrophilicity and Separation
- Stationary phase characteristics: Polar functional groups (e.g., amino, diol) bonded to silica. Larger pores improve diffusion for large molecules, while higher surface coverage provides more interaction sites.
- Hydrophobic mobile phase: High organic solvent content reduces solubility of polar compounds, enhancing interaction with the stationary phase.
- Gradient elution: Increasing water content enhances mobile phase polarity, reducing retention time for polar compounds.
- Molecular size and diffusion: Smaller molecules diffuse faster, increasing retention time. Molecular shape also influences interactions.
- Mobile phase pH: pH affects the charge state of polar compounds, altering electrostatic interactions with the stationary phase.
2.4 Applications
- Hydrophilic peptide separation: Suitable for highly polar peptides and proteins.
- Post-translational modification analysis: Used for enriching phosphorylated and glycosylated peptides.
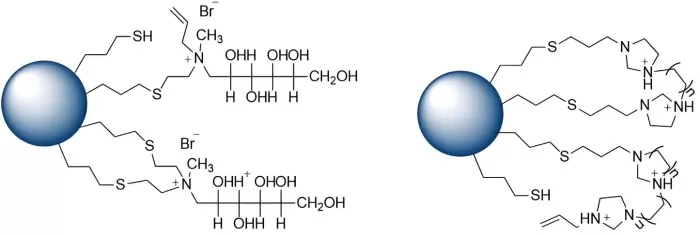
HILIC Column Packing
3. Ion-Exchange Chromatography (IEX)
3.1 Principle of IEX
IEX separates proteins based on surface charge differences. The stationary phase consists of charged resins (e.g., cation or anion exchangers), while the mobile phase is a buffer with varying ionic strengths. Charged proteins interact electrostatically with the stationary phase, and elution is achieved by changing ionic strength or pH.
3.2 Protein Separation Based on Charge
The core principle of IEX is electrostatic interaction between protein charges and ionic groups on the stationary phase. The charge state of proteins depends on amino acid composition and mobile phase pH. The separation process involves:
- Stationary and mobile phases: The stationary phase contains ionic groups (e.g., sulfonate for cation exchange, quaternary ammonium for anion exchange), while the mobile phase is a buffer with adjustable pH and ionic strength.
- Sample loading: The sample is dissolved in a buffer and injected into the column.
- Separation mechanism: Charged molecules interact with the stationary phase, while uncharged molecules are not retained. Cation-exchange chromatography (CEX): Separates positively charged molecules (e.g., under acidic conditions). Anion-exchange chromatography (AEX): Separates negatively charged molecules (e.g., under alkaline conditions).
- Elution methods: Changing pH: Adjusting pH alters protein charge, reducing interaction with the stationary phase. Changing ionic strength: Increasing salt concentration shields electrostatic interactions. Gradient elution: Gradually increasing salt concentration or changing pH elutes molecules with different charge densities.
3.3 Charge and Separation
- Stationary phase characteristics: Ionic groups (e.g., sulfonate, quaternary ammonium) determine interaction strength. Larger pores improve diffusion for large molecules, while higher surface coverage provides more adsorption sites.
- Mobile phase composition: pH and ionic strength affect protein charge and interaction with the stationary phase.
- Elution methods: Changing pH or ionic strength disrupts electrostatic interactions, enabling elution.
- Isoelectric point (pI): Proteins are positively charged below their pI and negatively charged above it.
4. Gel Permeation Chromatography (GPC) / Size-Exclusion Chromatography (SEC)
4.1 Principle of GPC/SEC
GPC/SEC separates molecules based on size exclusion. The stationary phase consists of porous particles, and molecules are separated based on their ability to enter the pores. Larger molecules elute first, while smaller molecules elute later.
4.2 Protein Separation Based on Size
The separation principle of GPC/SEC is based on molecular size exclusion. The separation process involves:
- Stationary and mobile phases: The stationary phase consists of porous particles (e.g., Sephadex, Sephacryl), while the mobile phase is a low-viscosity buffer (e.g., PBS).
- Sample loading: The sample is dissolved in the mobile phase and injected into the column.
- Separation mechanism: Larger molecules cannot enter the pores and elute first, while smaller molecules enter the pores and elute later.
- Elution order: Molecules elute in descending order of size.
4.3 Size and Separation
- Pore size selection: Small pores separate smaller molecules, while large pores separate larger molecules.
- Mobile phase: Low-viscosity buffers improve separation efficiency.
- Molecular size and shape: Larger molecules elute first, while smaller molecules elute later. Molecular shape also affects diffusion.
4.4 Applications
- Protein purification: Separates proteins by molecular weight.
- Polymer analysis: Determines molecular weight distribution.
- Desalting and buffer exchange: Separates biomacromolecules from small-molecule impurities.
5. Affinity Chromatography (AC)
5.1 Principle of AC
AC separates molecules based on specific interactions between target molecules and ligands on the stationary phase. Ligands (e.g., antibodies, enzyme substrates) bind specifically to target molecules, which are eluted by changing conditions (e.g., pH, ionic strength).
5.2 Protein Separation Based on Specific Interactions
The separation mechanism of AC involves specific binding between target molecules and ligands. The separation process involves:
- Stationary and mobile phases: The stationary phase consists of ligands (e.g., antibodies) bonded to porous particles, while the mobile phase is a low-salt buffer.
- Sample loading: The sample is dissolved in the mobile phase and injected into the column.
- Separation mechanism: Target molecules bind specifically to the ligands, while impurities are eluted. Elution is achieved by changing pH, ionic strength, or adding competitive ligands.
5.3 Specific Interactions and Separation
- Stationary phase characteristics: Ligands (e.g., antibodies) are bonded to porous particles. Larger pores improve diffusion for large molecules, while higher surface coverage provides more binding sites.
- Elution methods: Changing pH, ionic strength, or adding competitive ligands disrupts specific interactions, enabling elution.
5.4 Applications
- Protein purification: Purifies specific enzymes, antibodies, or receptors.
- Biomolecule separation: Separates nucleic acids, polysaccharides, etc.
- Drug development: Screens and purifies drug candidates with specific biological activities.
6. Technical Comparison
Reversed-Phase Chromatography (RPLC) and Hydrophilic Interaction Liquid Chromatography (HILIC) separate proteins based on hydrophobicity and hydrophilicity differences, respectively. RPLC is ideal for hydrophobic peptides and mass spectrometry compatibility, while HILIC excels in analyzing hydrophilic molecules and post-translational modifications, though both require precise mobile phase control. Ion-Exchange Chromatography (IEX) and Gel Permeation Chromatography/Size-Exclusion Chromatography (GPC/SEC) leverage charge and molecular size differences. IEX offers high capacity for charged proteins but demands strict pH/ionic strength optimization, whereas GPC/SEC preserves bioactivity without interactions but has lower resolution and longer separation times. Affinity Chromatography (AC) achieves highly selective purification via specific biomolecular interactions, ideal for isolating targets from complex matrices. However, it requires costly ligand customization and risks altering target activity during elution.
Comparison of different chromatography techniques
|
Technique |
Principle |
Advantages |
Limitations |
Applications |
|
RPLC |
Hydrophobicity |
High resolution, MS-compatible |
Low efficiency for hydrophobic macromolecules, organic gradients |
Peptide separation (MS prep), hydrophobic molecules |
|
HILIC |
Hydrophilicity |
Polar molecule analysis, PTM support |
High organic solvent risk, strict gradient control |
Hydrophilic peptides, PTM analysis |
|
IEX |
Charge differences |
High capacity, charge protein purification |
Requires pH/ionic optimization, complex charge challenges |
Charged protein purification, complex separation |
|
GPC/SEC |
Size exclusion |
No interaction, preserves bioactivity |
Low resolution, time-consuming, pore matching |
Size fractionation, desalting/buffer exchange |
|
AC |
Specific binding |
High specificity, complex sample handling |
High ligand cost, elution risks activity |
Target-specific purification (enzymes), drug screening |
References:
1. Field JK, Euerby MR, Haselmann KF, Petersson P. Investigation into reversed-phase chromatography peptide separation systems Part IV: Characterisation of mobile phase selectivity differences. J Chromatogr A. 2021 Mar 29;1641:461986. doi: 10.1016/j.chroma.2021.461986. Epub 2021 Feb 9. PMID: 33631703.
2. Lenčo J, Jadeja S, Naplekov DK, Krokhin OV, Khalikova MA, Chocholouš P, Urban J, Broeckhoven K, Nováková L, Švec F. Reversed-Phase Liquid Chromatography of Peptides for Bottom-Up Proteomics: A Tutorial. J Proteome Res. 2022 Dec 2;21(12):2846-2892. doi: 10.1021/acs.jproteome.2c00407.
3. Lämmerhofer M, Nogueira R, Lindner W. Multi-modal applicability of a reversed-phase/weak-anion exchange material in reversed-phase, anion-exchange, ion-exclusion, hydrophilic interaction and hydrophobic interaction chromatography modes. Anal Bioanal Chem. 2011 Jun;400(8):2517-30. doi: 10.1007/s00216-011-4755-3.
4. Milana MR, Denaro M, Arrivabene L, Maggio A, Gramiccioni L. Gel permeation chromatography (GPC) of repeatedly extruded polyethylene terephthalate (PET). Food Addit Contam. 1998 Apr;15(3):355-61. doi: 10.1080/02652039809374651. PMID: 9666895.
5. Rodriguez EL, Poddar S, Iftekhar S, Suh K, Woolfork AG, Ovbude S, Pekarek A, Walters M, Lott S, Hage DS. Affinity chromatography: A review of trends and developments over the past 50 years. J Chromatogr B Analyt Technol Biomed Life Sci. 2020 Nov 10;1157:122332. doi: 10.1016/j.jchromb.2020.122332.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


