Protein Secondary Structure Characterization: Principles and Applications
Proteins play a pivotal role in various biological processes, and their structural properties are fundamental to understanding their functions. Among different levels of protein structures, the secondary structure refers to the local folding and conformation of the polypeptide backbone, which significantly influences protein stability and interactions. The primary elements of protein secondary structures include α-helices, β-sheets, β-turns, and random coils. Characterizing protein secondary structures is crucial for deciphering protein functions and interaction mechanisms. This blog provides an in-depth overview of protein secondary structures and the common techniques used for their characterization, along with their principles, experimental steps, advantages, and limitations.
_1741574899_WNo_684d688.webp)
Four levels of protein structure (Gupta et al., 2017)
Introduction to Protein Secondary Structure
Protein secondary structure describes the local spatial arrangement of the polypeptide backbone without considering the side chain conformations. It mainly consists of four common elements:
- α-Helix: A right-handed spiral structure stabilized by hydrogen bonds between the amide hydrogen of one amino acid and the carbonyl oxygen of another amino acid four residues ahead.
- β-Sheet: A zigzag arrangement where adjacent polypeptide strands are connected by hydrogen bonds, forming either parallel or antiparallel configurations.
- β-Turn: A tight loop involving four amino acid residues that reverses the direction of the polypeptide chain, stabilized by hydrogen bonding.
- Random Coil: An irregular structure typically found in loop regions, serving as flexible connectors between α-helices and β-sheets.
Understanding these structural elements is essential for exploring protein functions, folding pathways, and interactions.
Techniques for Protein Secondary Structure Characterization
1. Circular Dichroism (CD) Spectroscopy
Principle: Circular Dichroism (CD) spectroscopy measures the differential absorption of left- and right-circularly polarized light by optically active protein molecules. Different secondary structure elements exhibit characteristic CD spectra in the far-UV region (190–250 nm), allowing quantitative estimation of α-helix, β-sheet, and random coil content.
Experimental Steps: Protein samples are typically dissolved in a buffer with low UV absorption. The CD spectrum is recorded by scanning the sample at different wavelengths, and the resulting signal is analyzed using computational algorithms to estimate the relative proportions of secondary structure components.
Advantages:
- Rapid and straightforward analysis
- Low sample consumption
- Suitable for monitoring structural changes under different conditions
- Can be used to study protein folding kinetics
Limitations:
- Provides only approximate structural information
- Requires high-purity samples
- Limited resolution for mixed or complex structures
- Sensitive to buffer composition and temperature
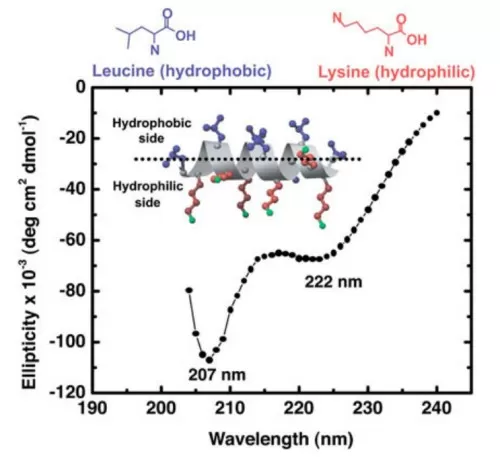
CD spectrum of 140 g/mL LK14 peptide in PBS buffer showing a typical -helical secondary structure (Mermut et al., 2006)
2. Fourier Transform Infrared (FTIR) Spectroscopy
Principle: FTIR spectroscopy detects vibrational modes of protein backbones, particularly amide I (1600–1700 cm⁻¹) and amide II (1500–1600 cm⁻¹) bands, which are highly sensitive to secondary structures. The position and shape of these absorption bands provide insights into α-helices, β-sheets, and random coils.
Experimental Steps: Protein samples are prepared in aqueous or non-aqueous solutions or embedded in thin films. The infrared spectrum is measured, and computational deconvolution techniques are applied to assign specific bands to secondary structure elements.
Advantages:
- Applicable to various sample forms (solid, liquid, and films)
- Provides detailed information on the chemical environment
- Suitable for complex mixtures
- Can be combined with temperature-dependent measurements to study protein stability
Limitations:
- High technical expertise required
- Costly instrumentation
- Interference from water absorption
- Requires careful sample preparation
3. Nuclear Magnetic Resonance (NMR) Spectroscopy
Principle: NMR spectroscopy detects the magnetic properties of atomic nuclei such as hydrogen, carbon, and nitrogen. By measuring chemical shifts, coupling constants, and nuclear Overhauser effects, NMR provides information on interatomic distances, dihedral angles, and dynamic properties, revealing protein secondary structures in solution.
Experimental Steps: Protein samples are dissolved in suitable solvents and placed in a magnetic field. NMR spectra are recorded using pulse sequences, and the resulting data are processed to obtain structural and dynamic information.
Advantages:
- Provides dynamic and structural information
- Suitable for solution-phase proteins
- Allows investigation of protein-ligand interactions
- Can detect conformational changes and protein dynamics at atomic resolution
Limitations:
- High sample concentration required
- Time-consuming data acquisition and analysis
- Limited to small or medium-sized proteins
- Requires advanced data interpretation skills
4. X-Ray Crystallography
Principle: X-ray crystallography determines the three-dimensional structure of proteins by analyzing the diffraction patterns of X-rays scattered by protein crystals. Though primarily used for tertiary structure determination, it also provides detailed insights into protein secondary structures.
Experimental Steps: Protein crystals are grown under controlled conditions, and X-ray diffraction data are collected. The diffraction patterns are processed using computational algorithms to reconstruct the electron density map and model the protein structure.
Advantages:
- High-resolution structural information
- Widely used for drug discovery and structural biology
- Provides atomic-level details of protein structures
- Suitable for large and complex proteins
Limitations:
- Requires high-quality crystals
- Time-consuming and expensive
- Not suitable for dynamic structures
- Crystallization may alter protein conformation
Transformative Applications: From Drug Discovery to Disease Mechanisms
The characterization of protein secondary structures has far-reaching implications across research and industry, driving innovations in medicine, biotechnology, and materials science.
1. Drug Development and Therapeutics
Understanding secondary structures is pivotal in designing peptide-based drugs and biologics. For example:
- Insulin analogs: Modifying β-sheet and α-helical regions has led to rapid-acting and long-lasting insulin formulations, improving diabetes management.
- Antimicrobial peptides: Engineering α-helical peptides with enhanced stability and membrane permeability offers new strategies to combat antibiotic-resistant bacteria.
- Protein misfolding diseases: Identifying β-sheet-rich amyloid fibrils in Alzheimer’s and Parkinson’s diseases has spurred the development of inhibitors targeting early aggregation intermediates.
2. Biomaterials and Tissue Engineering
Proteins with defined secondary structures are harnessed for their mechanical and functional properties:
- Silk fibroin: Rich in β-sheets, silk proteins are used in biodegradable sutures, wound dressings, and tissue scaffolds due to their strength and biocompatibility.
- Collagen mimics: Triple-helical collagen analogs are engineered for skin grafts and cartilage repair, mimicking the extracellular matrix.
3. Industrial Enzymes and Biocatalysis
Optimizing secondary structures enhances enzyme stability and activity under industrial conditions:
- Thermostable enzymes: Engineering α-helices and β-sheets in proteases and lipases improves their performance in detergents and biofuel production.
- Substrate specificity: Tailoring β-turns in active sites enables the design of enzymes for synthesizing chiral pharmaceuticals.
4. Disease Mechanisms and Diagnostics
Secondary structure analysis sheds light on pathological processes:
- Prion diseases: Misfolded β-sheet-rich prion proteins propagate neurodegenerative disorders like Creutzfeldt-Jakob disease.
- Cancer biomarkers: Aberrant secondary structures in oncogenic proteins (e.g., p53) serve as diagnostic markers and therapeutic targets.
5. Emerging Trends:
- AI-driven drug design: Machine learning models predict secondary structures from sequences, accelerating the discovery of novel therapeutics.
- High-throughput screening: Automated CD and FTIR platforms enable rapid structural analysis of protein libraries for drug candidates.
The Future of Protein Secondary Structure Characterization
As we look ahead, the field of protein secondary structure characterization is poised for transformative advancements, driven by technological innovations and interdisciplinary collaborations.
1. Integration of Experimental and Computational Approaches
Hybrid methods combining experimental data (e.g., CD, NMR) with computational modeling (e.g., molecular dynamics, machine learning) are revolutionizing structural biology:
- AlphaFold2: This AI tool predicts protein structures with unprecedented accuracy, including secondary structural elements, from amino acid sequences alone.
- Enhanced spectral analysis: Advanced algorithms deconvolute overlapping CD and FTIR signals, improving resolution and reliability.
2. Real-Time and In Situ Analysis
Emerging technologies enable dynamic and context-specific structural studies:
- Time-resolved spectroscopy: Captures transient folding intermediates during protein synthesis or ligand binding.
- Cryo-EM tomography: Visualizes secondary structures in cellular environments, bridging the gap between in vitro and in vivo studies.
3. Personalized Medicine and Precision Therapeutics
Secondary structure characterization is integral to tailoring treatments to individual patients:
- Protein-based diagnostics: Detecting structural biomarkers in blood or tissue samples enables early disease diagnosis.
- Custom biologics: Designing patient-specific protein therapeutics (e.g., monoclonal antibodies) with optimized stability and efficacy.
4. Sustainable Biotechnology
Harnessing secondary structure insights promotes eco-friendly innovations:
- Enzyme engineering: Designing robust enzymes for biodegrading plastics and pollutants.
- Plant-based proteins: Optimizing the secondary structures of plant proteins to mimic meat textures, supporting sustainable food systems.
Conclusion: Unlocking the Molecular Blueprint of Life
Protein secondary structure characterization is a cornerstone of modern molecular science, bridging the gap between sequence and function. From elucidating disease mechanisms to designing life-saving drugs, these techniques have transformed our understanding of biology and opened new frontiers in medicine and technology. While each method—CD, FTIR, NMR, and X-ray crystallography—has its strengths and limitations, their combined use provides a comprehensive view of protein architecture. As technologies evolve, from AI-driven predictions to real-time structural analysis, researchers are equipped to tackle increasingly complex biological questions.
The future holds immense promise, with applications ranging from personalized medicine to sustainable biotechnology. By mastering these techniques and embracing interdisciplinary innovations, scientists will continue to decode the molecular choreography of life, paving the way for breakthroughs that benefit humanity and the planet.
References:
Greenfield NJ. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc. 2006;1(6):2876-2890. doi:10.1038/nprot.2006.202
Jumper J, Evans R, Pritzel A, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583-589. doi:10.1038/s41586-021-03819-2
Barth A. Infrared spectroscopy of proteins. Biochim Biophys Acta. 2007;1767(9):1073-1101. doi:10.1016/j.bbabio.2007.06.004
Gupta R, Dey A, Vijan A, Gartia B (2017) In Silico Structure Modeling and Characterization of Hypothetical Protein YP_004590319.1 Present in Enterobacter aerogens. J Proteomics Bioinform 10: 152-170. doi: 10.4172/jpb.1000436
Mermut O, York RL, Phillips DC, McCrea KR, Ward RS, Somorjai GA. Directions in peptide interfacial science. Biointerphases. 2006;1(2):P5-P11. doi:10.1116/1.2194033


