Protein sample preparation tips: Serum or Plasma?
In proteomics research, the choice of blood sample is crucial. This article delves into the best choice between serum and plasma for proteomics, providing detailed tips and methods for sample preparation. Here are the main sections of this article:
1. Introduction to Blood Proteomics: Serum vs. Plasma
2. Plasma Preparation Protocol: Step-by-Step Guide
3. Serum Preparation Protocol: Step-by-Step Guide
4. Key Differences Between Plasma and Serum
5. Advantages of Using Plasma Over Serum in Proteomics
6. Characteristics of the Blood Proteome
7. Advanced Techniques for Blood Proteomics
8. Conclusion: Optimal Choice for Proteomic Analysis
1. Introduction to Blood Proteomics: Serum vs. Plasma
There is a proverbial saying that "the eyes are the windows to the soul." In medical research, blood serves as a conduit through which the entire body's circulatory characteristics and individual physiological functions are reflected, rendering it a window into understanding a patient's physical condition. Researchers glean valuable insights from the blood proteome. However, in the majority of proteomic studies, the focus lies not on analyzing the entire blood sample, but rather on dissecting the plasma or serum components.
Given the significant value of blood samples, the classic inquiries in blood proteomics persist: Should one opt for serum or plasma? How should samples be prepared? And what are the steps for conducting proteomic experiments? These questions were thoroughly explored in the article "Serum and Plasma Proteomics," published in Chemical Reviews in 2007. The article provides detailed insights into the rationale behind utilizing blood in proteomics, the distinctive features of blood proteins, and methodologies for their analysis.
2. Plasma Preparation Protocol: Step-by-Step Guide
Plasma is the supernatant portion of blood after all cellular components have been removed. It is obtained as follows:
1. Collect blood in a collection tube containing EDTA or sodium heparin.
2. Immediately mix gently by inverting the tube several times.
3. Centrifuge at 3000 rpm for 10 minutes at 4°C.
4. Transfer the supernatant (i.e., plasma) to a centrifuge tube.
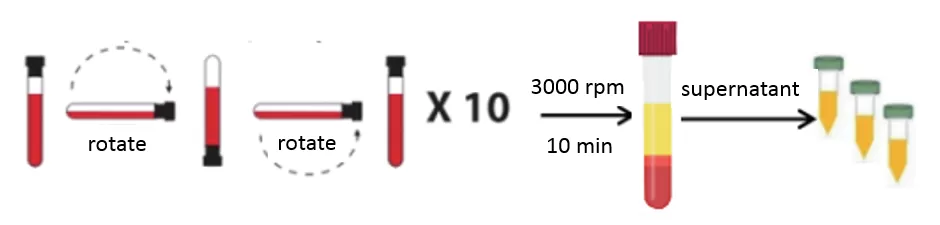
3. Serum Preparation Protocol: Step-by-Step Guide
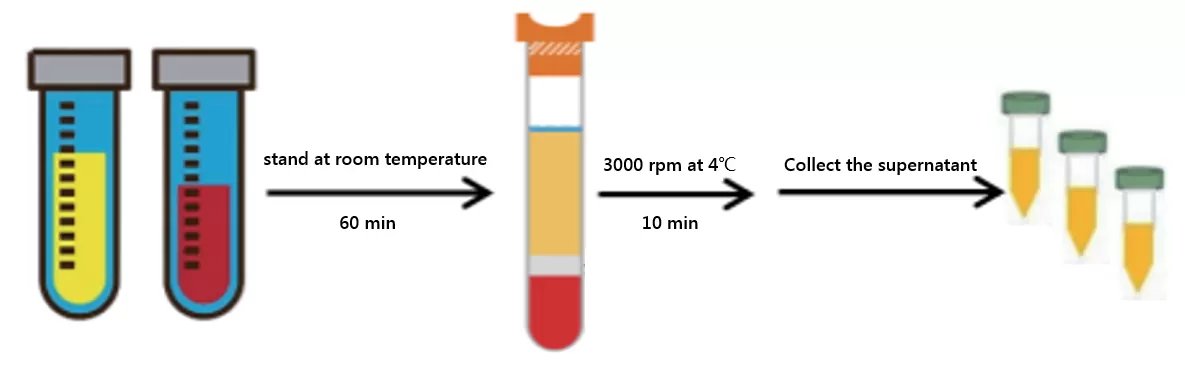
Serum is obtained from blood without anticoagulants and involves the removal of fibrin clots. The procedure is as follows:
1. Collect blood samples in tubes containing serum separation gel and coagulants (recommended brand: BD).
2. Allow the tubes to stand at room temperature for 60 minutes to facilitate coagulation (avoid agitation).
3. Centrifuge the tubes at 3000 rpm for 10 minutes at 4°C.
4. Transfer the supernatant (i.e., serum) to a centrifuge tube.
4. Key Differences Between Plasma and Serum
The coagulation process leads to essential differences between serum and plasma. The fibrin clot in plasma contains most of the fibrinogen, so when the clot is removed from serum, it results in a lower protein concentration compared to plasma—though the difference is only around 3-4%. Other proteins may also be lost due to specific or non-specific interactions with the fibrin clot. Traditionally, it has been thought that many coagulation factors are also removed during serum preparation. However, coagulation factors IX, X, XI, and VII/VIIa are found in serum. The main impact of the coagulation process is the removal of the fibrin clot, platelets, red blood cells, and white blood cells, as well as an increase in the concentration of certain proteins in serum. Some studies indicate that vascular endothelial growth factor (VEGF) levels in serum and plasma are influenced by platelets, but this is more pronounced in serum (Haleem J. Issaq, Zhen Xiao, and Timothy D. Veenstra, 2007).
5. Advantages of Using Plasma Over Serum in Proteomics
In summary, plasma offers certain advantages over serum for proteomics analysis: 1) Plasma sampling is easier, as the clotting and centrifugation times involved in serum sampling significantly impact the proteome; 2) Serum protein concentration is lower than that of plasma because some proteins are removed during the coagulation process; 3) Serum is more significantly influenced by platelets.
6. Characteristics of the Blood Proteome
Human plasma contains circulating proteins at a concentration of approximately 70 mg/ml, spanning an estimated range of 12 orders of magnitude. Blood samples encompass at least 10,000 distinct protein types, with around 3,000 classified as classical resident plasma proteins. High-abundance proteins such as albumin, immunoglobulins, transferrin, and fibrinogen constitute 97% to 99% of the total blood protein content, with concentrations in the mg/ml range. Proteins derived from secretions or leakages from various organs are diluted in peripheral blood and can reach concentrations as low as pg/ml, such as IL-6, IL-12, and tumor necrosis factor-alpha. These low-abundance proteins are often masked by higher-abundance proteins in proteomics analysis.
7. Advanced Techniques for Blood Proteomics
The dominance of high-abundance proteins in blood limits the efficiency of conventional proteomic research, constraining the detection throughput of blood proteins. Even with high-throughput proteomic techniques like TMT and DIA, most analyses detect only between 200 and 800 proteins, which is insufficient for researchers' needs.
To address the challenges posed by high-abundance proteins in blood proteomics, several advanced techniques have been developed, which can be classified into three main types: high-abundance protein depletion (non-antibody based), high-abundance protein depletion (antibody based), and magnetic bead enrichment of low-abundance proteins. A notable method for high-abundance protein depletion (non-antibody based) is the small-volume protein concentration kit (Bio-Rad). Representative methods for high-abundance protein depletion (antibody based) include the Multiple Affinity Removal System (Agilent), ProteoExtract™ High Abundance Protein Depletion Kit (Merck), and IgY-based polyclonal antibody protein removal products (Seppro). A prominent method for magnetic bead enrichment of low-abundance proteins is the nanographene magnetic bead adsorption technique.
8. Conclusion: Optimal Choice for Proteomic Analysis
Choosing between serum and plasma for proteomic analysis requires careful consideration of the specific research goals and the advantages and limitations of each sample type. By understanding the preparation protocols, key differences, and advanced techniques available, researchers can optimize their proteomic studies to achieve the most accurate and comprehensive results.
Read more:
· A Guide to Protein Database Selection
· MetwareBio Launches Proteomics Services
· What is Isoelectric Points of Amino Acids: Essential Calculations and Practical Applications
· Comparison and Application of Proteomic Technologies
· Demystifying Proteomics Research Strategies and Content in a Single Read
· Optimal Protein Database Selection: Insights from Experimental Data
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


