Protein Digestion Unveiled: In-Gel vs. In-Solution Techniques
What is Protein Digestion
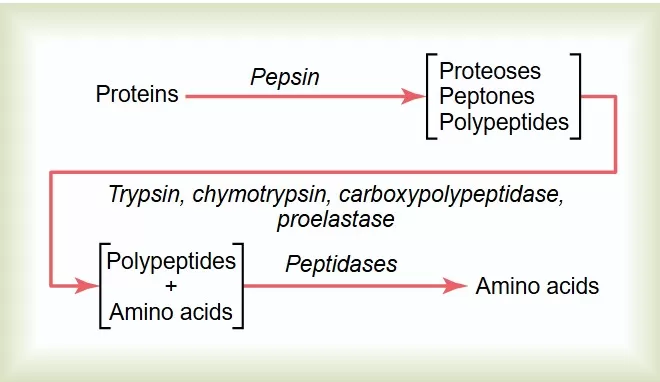
Protein digestion is the process of breaking down proteins into smaller peptides or individual amino acids. While this occurs naturally in biological systems, it can also be replicated in the lab using enzymatic reactions. In research, in-gel digestion is commonly employed for proteins separated by 2D electrophoresis, whereas in-solution digestion is typically used for LC-MS/MS analysis.
Protein Digestion in Gel
Protein gels are three-dimensional network structures formed by interactions between protein molecules. These interactions include hydrophobic forces, electrostatic attractions, and hydrogen bonds. Under specific conditions, such as heating or the addition of polysaccharides, proteins can form stable gels. However, in the gel state, the digestion rate of proteins is often slower due to the significant restrictions on their spatial structure. During gel formation, proteins undergo substantial changes in their secondary structure. Before gel formation, the content of α-helices typically decreases, while the content of β-sheets increases. After gel formation, both α-helices and β-sheets significantly increase, and random coil structures gradually disappear, leading to greater molecular order. These structural changes not only affect the physical properties of the proteins but may also have important implications for their digestibility.
Gel Formation and Protein Digestion
Different types of gels have varying impacts on the rate and efficiency of protein digestion. For example, in whey protein gels, heat treatment can induce faster digestion, which is related to the gel's microstructure. Additionally, the incorporation of gellan can result in more uniform dispersion of myofibrillar proteins, forming smaller protein aggregates that increase the surface area for contact with pepsin and trypsin, thereby enhancing protein digestion.
Moreover, the microstructure of protein gels can influence their digestive characteristics. Gels made with lower salt concentrations tend to be softer and have a more uniform microstructure, leading to higher lipid digestion rates, while firmer gels may delay digestion. These factors suggest that by modulating the microstructure and mechanical properties of gels, the digestion rate and extent of proteins can be effectively controlled.
External conditions such as temperature and pH significantly affect the formation and digestion of protein gels. For instance, elevated temperatures can promote interactions between protein molecules, enhance the exposure of hydrophobic groups, and lead to the formation of irreversible gel networks. In acidic environments, the spatial structure of proteins unfolds, exposing more binding sites for pepsin, which accelerates enzymatic hydrolysis.
In in vitro simulated digestion experiments, different types of gels exhibit distinct characteristics during digestion. For example, during gastric digestion of sheep cheese proteins, the digestion rate gradually increases over time, although there are variations among different types of casein. Additionally, whey proteins form irreversible gels when heated to certain temperatures, and the gel properties depend on factors such as protein concentration, solution pH, and the concentrations of calcium and sodium ions.
Applications of Gel Digestion Techniques
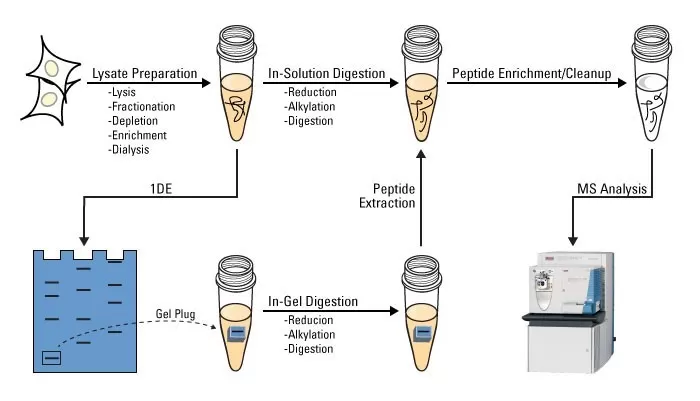
To enhance peptide recovery from gels and simplify digestion procedures, researchers have developed various efficient gel digestion methods. For instance, ProteaseMAX™ Surfactant effectively extracts peptides from gels, significantly improving peptide recovery rates and protein sequence coverage. This method not only increases experimental efficiency but also enhances the sensitivity and accuracy of mass spectrometry analyses.
Steps in Gel Digestion for Mass Spectrometry:
1. Sample Preparation: The protein samples to be analyzed are separated using gel electrophoresis, typically employing methods like SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) or 2D-PAGE (two-dimensional electrophoresis).
2. Gel Cutting: The target protein bands are carefully cut out with a knife and placed in an extraction solution for peptide extraction.
3. Protein Digestion: Proteases (such as trypsin) are added to the gel fragments, and digestion occurs under suitable temperature and buffer conditions for a specific duration.
4. Peptide Extraction: The digested peptides are extracted from the gel fragments, often using organic solvents like ethyl acetate or methanol.
Protein Digestion in Solution
In solution, the protein digestion process is relatively straightforward and rapid. Solution digestion is primarily used for LC-MS/MS analysis, providing high-resolution mass spectrometry data. Compared to gel digestion, solution digestion offers greater flexibility and reproducibility. Common methods involve using trypsin solutions for enzymatic digestion, with trifluoroacetic acid (TFA) used to terminate the reaction and recover the supernatant. The trypsin/Lys-C mixed enzyme is widely used for standard overnight digestion under non-denaturing conditions, enhancing protein quantification and improving the reproducibility of experimental results. Additionally, laser scattering techniques can measure changes in protein content in solution, aiding in the assessment of protein digestion levels.
Steps in Solution Digestion:
1. Sample Preparation: Protein samples are dissolved in an appropriate buffer, and the pH is adjusted to the desired range.
2. Enzyme Treatment: A suitable amount of protease (e.g., trypsin) is added, and digestion is performed overnight at an optimal temperature.
3. Peptide Extraction: Ultrasonic-assisted extraction is employed to release the digested peptides.
Conclusion
Protein digestion is a critical step in food processing and biomedical research. Whether conducted in gel or solution, precise control of conditions is essential for maximizing protein utilization and analytical accuracy. By gaining a deeper understanding of the mechanisms of protein digestion and their performance in different environments, we can better design and optimize food formulations and drug delivery systems, thereby enhancing the nutritional value and functionality of products.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


