What is Phosphoproteomics: Decoding the Protein Phosphorylation Landscape
Phospho-proteomics is transforming our understanding of cellular signaling, shedding light on the dynamic phosphorylation events that regulate key biological processes. Imagine being able to trace the intricate molecular switches that control cell growth, division, and communication—all in real-time. With phospho-proteomics, we can dive deep into the functional landscapes of proteins, unraveling how their modifications shape health, disease, and even therapeutic response. In this blog, we’ll explore the cutting-edge techniques that make this possible, and why they’re essential for unlocking the secrets of cellular function.
What is Phosphorylation Modification?
Protein phosphorylation is one of the most studied and significant post-translational modifications (PTMs). It typically involves the enzyme-catalyzed transfer of a phosphate group from ATP to the side chain of a protein's amino acid, converting ATP into ADP. This process is usually reversible, with kinases catalyzing the addition of the phosphate group, and phosphatases removing it. Often referred to as a "molecular switch," phosphorylation plays a key role in regulating protein activity.
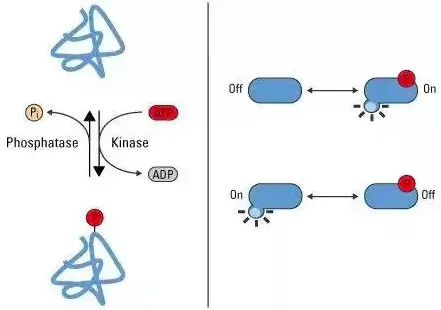
Interesting Discovery of Phosphorylated Proteins
In 1883, German chemist Olof Hammarsten first discovered that casein, the main protein in milk, contained phosphorus in addition to the five known elements (C, N, O, H, and S). This marked the first identification of a phosphorylated protein in history.
In 1954, Eugene Kennedy found that rat liver homogenate could transfer a phosphate group from ATP to casein, leading to the first definition of a kinase ("protein phospho kinase"). However, Kennedy remained puzzled by his discovery, wondering why a liver protein would phosphorylate a milk protein, as they seemed unrelated. This doubt led him to abandon his research on phosphorylation and shift his focus to lipid studies.
Years later, as the critical biological role of phosphorylation became clear, Kennedy regretted his decision, famously reflecting, "I dropped the study of protein kinases and, like the base Indian, cast a pearl away, else richer than all his tribe!"
In 1992, Fischer and Krebs were awarded the Nobel Prize for their work demonstrating that phosphorylation is a major mechanism of biological regulation.
Common Sites of Phosphorylation
In eukaryotes, phosphorylation typically occurs on serine, threonine, and tyrosine residues, as their side chains contain hydroxyl groups capable of accepting phosphate groups. Among these, serine phosphorylation is the most common, followed by threonine and tyrosine, which is less frequent. These phosphorylation events play crucial roles in cell signaling, regulating protein activity, stability, subcellular localization, or interactions with other proteins.
In addition to serine, threonine, and tyrosine, other amino acid residues that can undergo phosphorylation include:
1. Histidine (H): Phosphorylation of histidine is found in both prokaryotes and eukaryotes and plays a key role in signal transduction and metabolic regulation. It has unique chemical properties, including the presence of isomers and instability.
2. Arginine (R): Under certain conditions, arginine residues can be phosphorylated, potentially influencing signal transduction.
3. Cysteine (C): Cysteine phosphorylation, more common in prokaryotes, can affect protein structure and function.
4. Aspartic Acid (D) and Glutamic Acid (E): Phosphorylation of these residues is common in bacteria and involved in metabolism and signaling.
5. Lysine (K): Although less common, lysine residues can also undergo phosphorylation in specific contexts.
These phosphorylation events are key regulatory mechanisms across various biological systems, critical for controlling numerous cellular processes. Mass spectrometry advancements now enable researchers to identify phosphorylation sites on proteins, offering deeper insights into protein function and cell signaling pathways.
Functions of Protein Phosphorylation
Protein phosphorylation is a key post-translational modification (PTM) that plays numerous vital roles within cells. Some of its main functions include:
1. Regulating Protein Activity: Phosphorylation can alter protein conformation, modulating its activity. Many enzymes, for instance, are activated or inhibited by their phosphorylation status.
2. Controlling Signal Transduction: Phosphorylation is central to cellular signaling pathways, transmitting signals from activated cell surface receptors to intracellular targets and triggering biological responses.
3. Cell Cycle and Division Regulation: Phosphorylation controls key proteins in the cell cycle, ensuring proper progression through cell division.
4. Influencing Protein Stability and Degradation: Phosphorylation can signal for ubiquitination and subsequent proteasomal degradation, impacting protein stability and lifespan.
5. Regulating Subcellular Localization: Phosphorylation can affect protein interactions with the cytoskeleton or other structures, altering their intracellular distribution.
6. Gene Expression Regulation: By modifying transcription factors, phosphorylation can enhance or inhibit their ability to bind DNA, thereby controlling gene expression.
7. Modulating Protein-Protein Interactions: Phosphorylation can create or disrupt interfaces for protein interactions, affecting complex formation and function.
8. Metabolic Regulation: Phosphorylation adjusts enzyme activity in metabolic pathways to meet cellular energy needs and metabolic status.
9. Role in Disease: Aberrant phosphorylation is linked to diseases like cancer, diabetes, and neurodegenerative disorders, making it a key target for drug development.
10. Adaptive Response: Cells use phosphorylation to adapt to environmental changes, such as oxidative stress, nutrient availability, and pathogen attack.
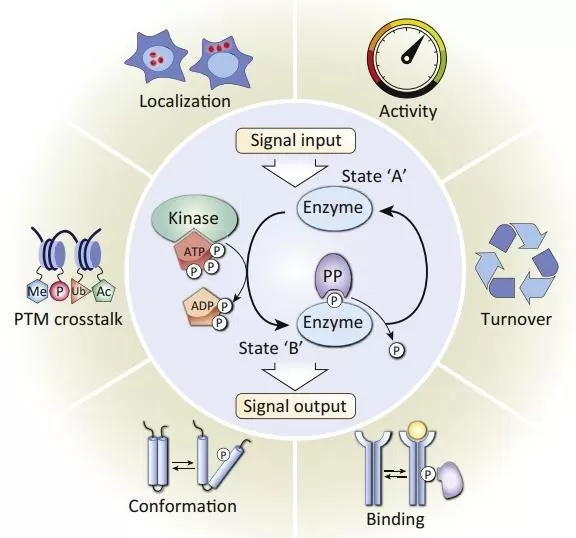
Applications of Phosphoproteomics
Phosphoproteomics, a key branch of proteomics, focuses on studying protein phosphorylation at a global scale to understand its role in biological processes. Utilizing mass spectrometry with specific sample preparation and enrichment methods, phosphoproteomics enables both qualitative and quantitative analysis of phosphorylated proteins within cells. Key applications include:
1. Identifying New Phosphorylation Sites: Comprehensive analysis of phosphorylated proteins helps uncover new phosphorylation sites and their potential biological functions.
2. Key Proteins in Signaling Pathways: Phosphoproteomics identifies crucial proteins in signaling pathways, providing insights into cellular signaling mechanisms.
3. Phosphorylation Abnormalities in Disease: Phosphoproteomics reveals phosphorylation abnormalities linked to diseases like cancer and inflammation, aiding in diagnostics and therapeutic targeting.
4. Drug Mechanism Studies: Phosphoproteomics helps understand the effects of drugs on protein phosphorylation, revealing mechanisms of drug action and efficacy.
Advances and Challenges in Phosphoproteomics
Recent advancements in phosphoproteomics have been driven by improved enrichment and separation methods for phosphorylated peptides. Various enrichment techniques, such as SH2 superdomains, enhance efficiency in low-abundance samples like tyrosine-phosphorylated proteins. Mass spectrometry technologies, including ESI, MALDI, and CID, have further advanced the detection of phosphoproteomes.
Phosphoproteomics has seen significant progress in disease research, notably in breast cancer, where it has uncovered new biomarkers and phosphorylation profiles for key proteins like MEK1, MEK2, and ERK1/2. In triple-negative breast cancer, phosphoproteomics aids in distinguishing mitotic stages of tumor cells.
The field also extends to plant biology, where phosphoproteomics is used to study molecular switches in signal transduction. Research groups have developed methods to efficiently purify phosphoproteomes from various plants, identifying around 11,000 phosphorylation sites with single-needle mass spectrometry.
Despite these advances, challenges remain. Phosphorylation is transient and reversible, demanding precise sample collection and preservation. Phosphorylated proteins are often low in abundance, requiring specific enrichment techniques. Furthermore, the negative charge introduced by phosphorylation complicates ionization during mass spectrometry, increasing analytical complexity.
Reference
Humphrey, S. J., James, D. E., & Mann, M. (2015). Protein Phosphorylation: A Major Switch Mechanism for Metabolic Regulation. Trends in endocrinology and metabolism: TEM, 26(12), 676–687. https://doi.org/10.1016/j.tem.2015.09.013
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


