Peptide Sequencing: Methods, Applications, and Advances in Proteomics
Peptide sequencing is a crucial technique in modern proteomics, playing a pivotal role in deciphering the structure and function of proteins. Proteins are composed of amino acids arranged in specific sequences, which dictate their folding, stability, and biological activity. Accurately determining these sequences allows scientists to better understand protein interactions, disease mechanisms, and potential therapeutic targets. Over the years, peptide sequencing methods have evolved significantly, from traditional chemical degradation techniques to sophisticated mass spectrometry-based approaches. These advancements have not only increased sequencing efficiency but also enabled high-throughput and large-scale proteomic analyses. This article explores the significance of peptide sequencing, the methodologies employed, and the latest innovations shaping the field.
The Importance of Peptide Sequencing in Biological Research
Peptide sequencing provides fundamental insights into the molecular mechanisms of life. By determining the precise order of amino acids in a peptide or protein, researchers can unravel the intricate relationships between protein structure and function. This knowledge is essential for studying protein-protein interactions, as many biological processes, including enzyme activity, signal transduction, and cellular communication, depend on proteins recognizing and binding to specific partners. Moreover, peptide sequencing plays a crucial role in disease research. Many pathological conditions, such as cancer, neurodegenerative disorders, and metabolic diseases, arise due to mutations or misfolding in protein structures. Identifying these aberrant sequences can help in diagnosing diseases, understanding their progression, and developing targeted therapies. In addition, peptide sequencing is indispensable in drug discovery, as many pharmaceuticals, including peptide-based drugs and monoclonal antibodies, rely on precise sequence information for their design and optimization.
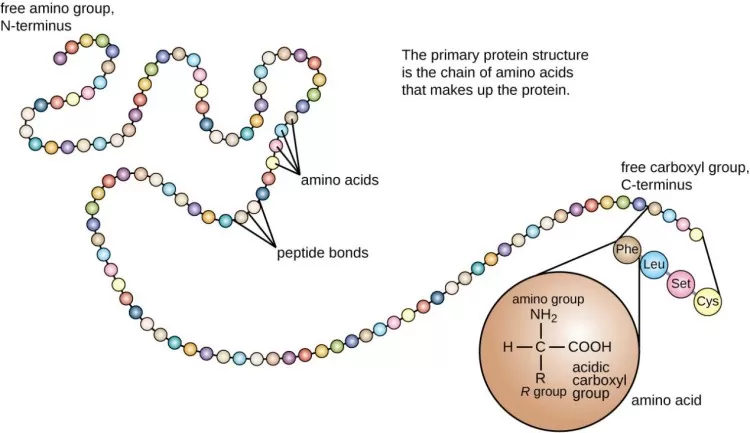
The formation of a peptide
Methods of Peptide Sequencing
Over the decades, several techniques have been developed to determine peptide sequences with increasing accuracy and efficiency. Each method has its own strengths and limitations, making it suitable for different applications depending on the complexity of the sample and the level of detail required.
Edman Degradation: A Classical Chemical Approach
Edman degradation is one of the earliest methods developed for peptide sequencing. This technique sequentially removes amino acids from the N-terminal end of a peptide, allowing them to be identified one at a time. The process involves reacting the peptide with phenyl isothiocyanate, which forms a cyclic derivative with the N-terminal amino acid. This derivative is then selectively cleaved and identified using chromatography. The procedure repeats iteratively, revealing the sequence of the peptide step by step.
Despite its precision, Edman degradation has certain limitations. It is most effective for short peptides, typically those under 50 amino acids in length, as the efficiency of the process decreases with longer sequences. Additionally, the sample must be highly purified, as impurities can interfere with the chemical reactions. Despite these constraints, Edman degradation remains a valuable tool for sequencing simple peptides and validating results obtained from other methods.
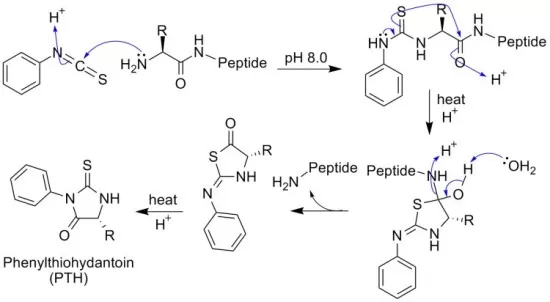
The principle of Edman degradation
Mass Spectrometry: A High-Throughput and Sensitive Approach
Mass spectrometry (MS) has revolutionized peptide sequencing by offering a rapid, high-throughput method for analyzing complex peptide mixtures. Unlike Edman degradation, which sequentially determines the sequence, MS measures the mass-to-charge ratio (m/z) of peptide fragments, allowing for sequence reconstruction. The most common technique used in peptide sequencing is tandem mass spectrometry (MS/MS), which involves fragmenting peptides into smaller ions and analyzing their mass spectra.
A significant advantage of MS-based sequencing is its ability to analyze peptides within complex biological samples, making it suitable for proteomics research, biomarker discovery, and disease diagnostics. It also provides information about post-translational modifications (PTMs), which are critical for understanding protein function. However, MS data interpretation can be challenging, requiring sophisticated computational tools to reconstruct peptide sequences accurately. Furthermore, the technique relies on high-resolution instrumentation, which can be costly and requires expertise in operation and data analysis.
De Novo Sequencing: Deciphering Novel Peptide Sequences
De novo sequencing is a specialized approach used when no reference sequence is available. Unlike database-dependent MS techniques, de novo sequencing relies on fragmentation patterns generated in MS/MS to directly deduce peptide sequences. This method is particularly useful for identifying new or modified proteins that may not exist in protein sequence databases.
One of the key advantages of de novo sequencing is its ability to characterize previously unknown proteins, making it essential in exploratory research and drug discovery. However, it is computationally intensive, requiring advanced algorithms to analyze fragment spectra accurately. The accuracy of de novo sequencing also depends on the quality of mass spectrometry data, with higher-resolution instruments providing more reliable results.
Next-Generation Sequencing (NGS) and Its Role in Peptide Analysis
Although NGS is primarily associated with DNA and RNA sequencing, it has been adapted for proteomic research through transcriptome sequencing. By sequencing mRNA, researchers can predict the amino acid sequences of peptides encoded by genes. This approach provides a complementary method for peptide identification, especially in large-scale proteomics studies.
NGS offers high throughput and scalability, enabling the analysis of vast numbers of peptides simultaneously. However, it does not directly sequence proteins but rather infers their sequences from genetic information. Therefore, its accuracy depends on the completeness and quality of the transcriptomic data. To validate predicted sequences, researchers often combine NGS with MS-based peptide sequencing.
Applications of Peptide Sequencing in Biomedical Research
The ability to sequence peptides has profound implications across various fields of biomedical research. In proteomics, peptide sequencing is essential for identifying and quantifying proteins in different biological systems. By comparing peptide sequences in healthy and diseased tissues, researchers can discover biomarkers that aid in early disease detection and prognosis. This is particularly important in cancer research, where altered protein expression patterns serve as diagnostic indicators and therapeutic targets.
In drug development, peptide sequencing facilitates the design of peptide-based drugs and monoclonal antibodies. Understanding the sequences of bioactive peptides allows researchers to modify their structures for enhanced stability, efficacy, and specificity. Moreover, sequencing plays a crucial role in vaccine development, where identifying immunogenic peptides helps in designing effective vaccines against infectious diseases.
Peptide sequencing also contributes to personalized medicine, where individual variations in protein sequences are analyzed to tailor treatments to specific patients. By identifying genetic mutations or modifications in peptide sequences, clinicians can develop targeted therapies that maximize therapeutic efficacy while minimizing adverse effects.
Recent Innovations and Future Directions in Peptide Sequencing
With continuous advancements in technology, peptide sequencing is becoming more efficient and precise. High-resolution mass spectrometry instruments, such as Orbitrap and Time-of-Flight (TOF) analyzers, have significantly improved sequence accuracy and sensitivity. These technologies enable the detection of low-abundance peptides and subtle modifications that were previously undetectable.
Artificial intelligence (AI) and machine learning are also transforming peptide sequencing. AI-driven algorithms enhance de novo sequencing accuracy, automate data analysis, and improve the interpretation of complex mass spectrometry data. By integrating AI with proteomics, researchers can uncover novel peptide sequences and protein interactions at an unprecedented scale.
Another exciting development is the integration of multiple sequencing techniques. Hybrid approaches combining Edman degradation, mass spectrometry, and bioinformatics allow for more comprehensive peptide analysis. Additionally, single-cell proteomics is emerging as a frontier in the field, enabling researchers to study peptide sequences at the level of individual cells, which has profound implications for cancer research and developmental biology.
Reference
1. Edwards, D. P., & Giddings, M. D. (2023). "Recent advances in peptide sequencing methods." Journal of Proteome Research, 22(4), 951-965.
2. Smit, D. J., & Li, H. (2022). "Mass spectrometry in proteomics: A review of recent developments." Trends in Analytical Chemistry, 148, 1-9.
3. Zhang, Y., & Sun, Y. (2021). "From Edman degradation to high-throughput proteomics: The evolution of peptide sequencing." Proteomics, 21(10), 2208-2221.
4. Wang, X., & Zhang, Z. (2020). "De Novo peptide sequencing with high-resolution mass spectrometry." Mass Spectrometry Reviews, 39(3), 214-230.


