O-Linked Glycosylation Uncovered: Processes, Health Impacts, and Research Methods
Glycosylation is a type of post-translational modification in which carbohydrate chains are covalently attached to specific amino acid residues. Based on the different types of glycopeptide chains, glycosylation can be classified into several categories: (1) N-glycosylation; (2) O-glycosylation; (3) C-glycosylation; (4) glycosylphosphatidylinositol (GPI) anchoring; (5) O-linked glycosylation (O-GlcNAcylation); and (6) others. Notably, O-linked glycosylation lacks fixed amino acid sequence features, making it complex and difficult to predict. Today, we will uncover the intricacies of O-linked glycosylation.
What is O-Linked Glycosylation
O-linked glycosylation is a crucial post-translational modification process catalyzed by various glycosyltransferases, which add single sugar residues to serine, threonine, tyrosine, and hydroxylysine sites until a complete glycan structure is formed. The different glycosyltransferases involved are primarily members of the N-acetylgalactosaminyltransferase (GalNAc-T) family, which includes over 20 distinct enzymes. Common monosaccharides involved in this process include N-acetylgalactosamine (GalNAc), galactose (Gal), N-acetylglucosamine (GlcNAc), and fucose (Fuc), among others.
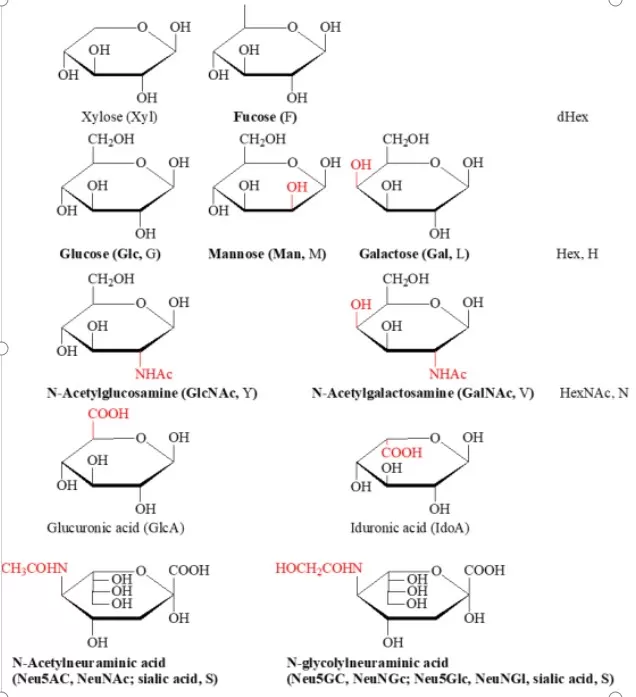
O-linked glycosylation can be categorized into mucin-type and non-mucin-type based on the types of monosaccharide residues connected to the amino acid sites. In mucin-type O-glycosylation, the monosaccharide residue linked to the protein is N-acetylgalactosamine (O-GalNAc), while in non-mucin-type O-glycosylation, the primarily linked monosaccharide residue is N-acetylglucosamine (O-GlcNAc).
Detailed Process of O-Linked Glycosylation
1. Substrate Recognition and Sugar Transfer: The process begins with glycosyltransferases, specifically members of the N-acetylgalactosaminyltransferase family, recognizing and binding to the hydroxyl group of specific amino acid residues on the target protein. Under the catalysis of the glycosyltransferase, a carbohydrate chain (typically composed of multiple monosaccharides) is transferred to the hydroxyl oxygen atom of the target amino acid residue.
2. Extension and Processing of the Carbohydrate Chain: Once the initial sugar is added, other glycosyltransferases can catalyze the addition of additional sugars. In the Golgi apparatus, the initial O-linked glycosylation structure undergoes a series of processing and extension steps, incorporating additional sugar molecules, such as xylose, glucose, fucose, and N-acetylglucosamine, as illustrated in Figure 1. These processing steps can yield a complex and diverse carbohydrate chain structure, varying from a single GalNAc to over 20 sugar residues.
3. Further Processing and Modification: After the carbohydrate chain is attached to the protein, further processing and modifications may occur, including elongation, branching, and deacetylation of the sugar chains. These modifications contribute to the formation of glycoproteins with specific functions and properties.
4. Maturation and Secretion: The processed and modified glycoproteins eventually mature within the Golgi apparatus and are released into the extracellular space or transported to other organelles through the Golgi's secretory function.
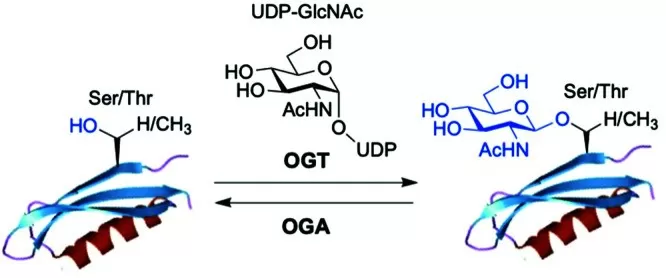
Roles of O-Linked Glycosylation in Human Diseases
O-linked glycosylation is a crucial post-translational modification that plays significant roles in various biological processes, and its dysregulation is associated with several human diseases. Here are some key areas where O-linked glycosylation impacts human health:
- Cancer: O-linked glycosylation is often altered in cancer cells, influencing tumor progression, metastasis, and immune evasion. Specific glycoproteins, such as mucins, exhibit increased O-linked glycosylation in many cancers, which can enhance cell proliferation and survival while suppressing apoptosis. For instance, the aberrant glycosylation patterns of mucin 1 (MUC1) are linked to breast cancer, contributing to its aggressive characteristics and poor prognosis.
- Inflammatory Diseases: O-linked glycosylation modulates the immune response by affecting the interaction between immune cells and cytokines. In chronic inflammatory diseases, such as rheumatoid arthritis and inflammatory bowel disease, changes in O-linked glycosylation patterns can lead to the production of pro-inflammatory cytokines and altered immune cell function, perpetuating the inflammatory response.
- Neurodegenerative Disorders: In diseases like Alzheimer's and Parkinson's, O-linked glycosylation plays a role in protein aggregation and toxicity. For example, the glycosylation state of tau protein affects its aggregation propensity, influencing the development of neurofibrillary tangles in Alzheimer's disease. Additionally, alterations in O-GlcNAcylation have been implicated in synaptic dysfunction and cognitive decline.
- Metabolic Disorders: O-linked glycosylation is closely linked to metabolic regulation, particularly in conditions such as obesity and diabetes. The modification affects insulin signaling and glucose metabolism. For instance, changes in O-GlcNAcylation of key metabolic enzymes can disrupt insulin signaling pathways, contributing to insulin resistance and type 2 diabetes.
- Cardiovascular Diseases: Alterations in O-linked glycosylation have been associated with cardiovascular diseases, influencing the stability and function of proteins involved in vascular homeostasis. For instance, changes in glycosylation of the low-density lipoprotein (LDL) receptor can impact cholesterol metabolism and atherogenesis.
- Infectious Diseases: Pathogens often exploit O-linked glycosylation to evade the immune system or enhance their pathogenicity. Certain viruses and bacteria can modify their surface proteins through glycosylation, allowing them to mimic host cells and avoid immune detection. For example, the glycosylation of the HIV envelope protein is critical for its interaction with host cell receptors and immune evasion.
Common Research Methods of O-Linked Glycosylation
O-linked glycosylation plays a crucial role in the structure and function of proteins. Common techniques for detecting protein glycosylation modifications include lectin affinity techniques, nuclear magnetic resonance (NMR), and mass spectrometry, with mass spectrometry being the most prevalent.
1. Lectin Affinity Techniques: Lectins can specifically adsorb and bind to one or more glycoproteins, facilitating the enrichment of O-linked glycosylated proteins. For instance, plant lectins (such as wheat germ agglutinin, WGA) exhibit a strong affinity for O-linked glycan chains, allowing for the enrichment of O-linked glycoproteins or glycopeptides.
2. Nuclear Magnetic Resonance (NMR): NMR techniques can determine the conformation, linkage positions, branching, and micro-diversity of sugar chains. While NMR can analyze structural features of glycan chains, the overlapping signal peaks for sugars make interpretation challenging, and multidimensional NMR requires milligram-scale samples, which is difficult to obtain from trace glycan chains in most glycan complexes.
3. Mass Spectrometry: The process begins with sample preparation, which involves extracting the target proteins and subjecting them to appropriate treatments, including purification, digestion (using specific proteases to cleave proteins into peptide segments), and enrichment of the peptides. Since O-linked glycosylated peptides may be present at low abundance in samples, specific enrichment techniques are typically employed to enhance detection sensitivity. These enrichment techniques include hydrophilic interaction chromatography (HILIC), metal affinity chromatography (such as IMAC or MOAC), and chemical enrichment methods (such as hydrazine chemistry or boronic acid chemistry). The enriched glycopeptides are then analyzed using a mass spectrometer, which separates and detects ionized glycopeptides based on their mass-to-charge ratio (m/z). During mass spectrometry analysis, glycopeptides are ionized and fragmented in a vacuum to obtain structural information, employing common fragmentation modes such as collision-induced dissociation (CID), higher-energy collision dissociation (HCD), electron capture dissociation (ECD), or electron transfer dissociation (ETD). These fragmentation modes help generate fragment ions that provide structural insights into both the glycan chain and peptide segments. Finally, by integrating mass spectrometry data with software analysis, one can determine the expression levels and specific sites of O-linked glycosylation, as well as elucidate the composition and structure of the glycan chains.
Reference:
Saha A, Bello D, Fernández-Tejada A. Advances in chemical probing of protein O-GlcNAc glycosylation: structural role and molecular mechanisms. Chem Soc Rev. 2021 Sep 20;50(18):10451-10485. doi: 10.1039/d0cs01275k. PMID: 34338261; PMCID: PMC8451060.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


