Non-oxidative pentose phosphate pathway controls regulatory T cell function
We are very pleased to share the autoimmune disease study published in Nature Metabolism Journal. The authors of “Non-oxidative pentose phosphate pathway controls regulatory T cell function by integrating metabolism and epigenetics” utilized our proprietary energy targeted metabolism detection and reported 50+ energy metabolites. The aim of this study was to explore how the non-oxidized pentose phosphate pathway (PPP) regulates Treg function to prevent autoimmunity.
Regulatory T cells (Treg), as a master suppressor of the immune system, limit immune-derived inflammation by preventing effector T cells from exhibiting excessive activation, proliferation or cytokine production. When Treg cells switch from quiescence to rapid proliferation upon antigen stimulation diseases, metabolic conversion is required to generate energy and macromolecules from nutrients to match functional demands. Treg cells have been reported to rely more on fatty acid β-oxidation and oxidative phosphorylation (OXPHOS) to support their function. However, the difficulties of targeting the X-chromosome-encoded transcription factor FOXP3 or these canonical metabolic pathways emphasize the need to explore new mechanisms to control Treg function.
Being a fundamental component of cellular metabolism, the non-oxidative pentose phosphate pathway (PPP) not only bridges glycolytic intermediates and ribose-5-phosphate (R5P), but also regulates glucose-derived carbon feeding into the tricarboxylic acid cycle (TCA cycle) in metabolic tissues. The non-oxidative PPP originating from ribulose-5-phosphate, a terminal metabolite in oxidative PPP, consists of several reversible reactions. Transketolase (TKT) stands out among the non-oxidative PPP enzymes by catalysing two reversible reactions, including the conversion of Xu5P and R5P to glyceraldehyde-3-phosphate (G3P) and sedoheptulose-7-phosphate (S7P) as well as the conversion of Xu5P and erythrose-4-phosphate to G3P and fructose-6-phosphate (F6P). By constructing a Treg-specific TKT-deficient mouse model, the authors found that the TKT knockout blocked the immunosuppressive function of Treg cells, leading to severe autoimmune diseases, which reveals a pivotal role of non-oxidized PPP in maintaining Treg function.
Comparing levels of amino acid metabolism in Treg cells of WT and cKO mice aged 2 to 3 weeks using LC-MS/MS, the author discovered that non-oxidative PPP balances fuel catabolism in Treg cells. TKT deficiency significantly reduced amino acid levels in Treg cells. The [13C6] isoleucine tracing assay indicated that more amino acids entered the TCA cycle in cKO Treg cells. The absence of TKT in Treg cells caused metabolic remodeling, such as activation of amino acid catabolism, activation of fatty acid metabolism, and reduction of glucose catabolism.
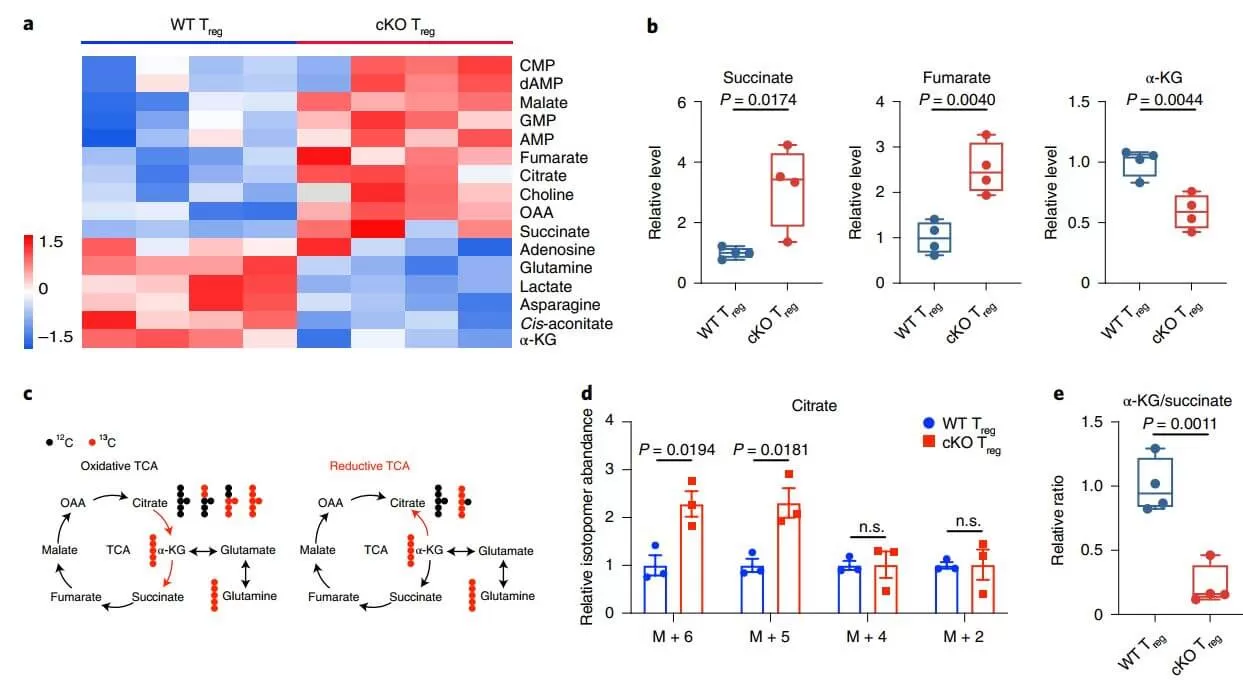
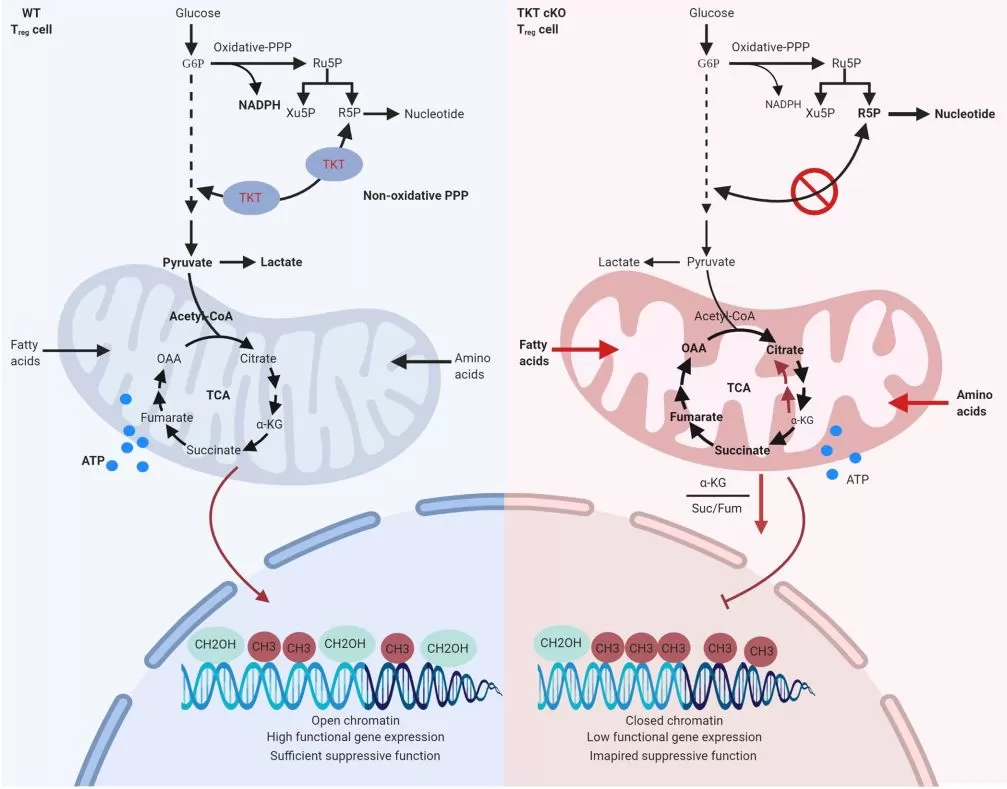
TCA cycle intermediates regulate epigenetic homeostasis in cancer and immune cells. Performing targeted metabolomics towards central metabolism, especially the TCA cycle in WT and cKO Treg cells from 2- to 3-week-old mice with mild inflammation, the authors found that the non-oxidative PPP plays a critical role in dividing carbon flows between glycolysis and de novo nucleotide biosynthesis. Limited lactate production and increased nucleotide biosynthesis could be observed in TKT-deficient Treg cells. Taken together, reduced glycolysis and enhanced oxidative stress induced by TKT deficiency triggers excessive fatty acid and amino acid catabolism, resulting in uncontrolled oxidative phosphorylation and impaired mitochondrial fitness. Reduced α-KG levels as a result of reductive TCA cycle activity leads to DNA hypermethylation, thereby limiting functional gene expression and suppressive activity of TKT-deficient Treg cells.
In conclusion, the non-oxidative PPP serves as a metabolic gatekeeper to balance Treg catabolism and transcriptome and epigenetic programmes, which specifically orchestrates Treg function and activates Treg differentiation.
Nat Metab. 2022 May; 4(5):559-574. doi: 10.1038/s42255-022-00575-z.
Non-oxidative pentose phosphate pathway controls regulatory T cell function by integrating metabolism and epigenetics
Conclusion
This study provides us with important insights into the key role of the non-oxidative pentose phosphate pathway in regulating Treg cell function. By revealing the profound effects of TKT deficiency on Treg cell metabolism and function, this study not only expands our understanding of the metabolic mechanisms of Treg cells, but also provides new potential targets for the treatment of autoimmune diseases. We hope that this discovery will promote future research and bring more possibilities for the development of targeted treatment options.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


