How Multi-Omics is Transforming Plant Research: Lessons from Tomato Studies
Over the last two decades, omics technologies—including genomics, transcriptomics, proteomics, and metabolomics—have transformed how we study complex biological systems. These cutting-edge tools enable researchers to decode genetic blueprints, analyze protein functions, and map metabolic pathways with unparalleled precision. As a result, the applications of multiple omics technologies span medicine, biotechnology, and agriculture, driving innovation in fields like crop improvement and sustainable farming.
In the field of plant research, multi-omics is a game-changer. By linking genetic information with biochemical processes, multi-omics provides a comprehensive understanding of traits like stress resistance, growth optimization, and crop yield enhancement. This powerful approach is helping researchers tackle critical challenges such as improving climate resilience and ensuring global food security.
This blog delves into the transformative potential of multi-omics by focusing on tomato research. Through three key applications—enhancing tomato color traits, boosting stress tolerance, and decoding plant development—we’ll explore how multi-omics is revolutionizing crop science and paving the way for more sustainable and efficient agricultural practices.
Unlocking Tomato Fruit Color Research with Multi-Omics
Tomato color is a key indicator of quality, ripeness, and nutritional value, directly influencing consumer preferences and marketability. Vibrant red tomatoes, rich in lycopene, are particularly prized for their health benefits, making color a vital factor for growers and suppliers aiming to boost market value and competitiveness. The genetic and biochemical pathways controlling tomato color are complex, with pigments like carotenoids and flavonoids playing crucial roles. These traits are regulated by a network of genes, metabolic processes, and environmental factors. Traditional breeding has struggled to fully unravel these complexities. However, by applying multi-omics—combining genomics, transcriptomics, metabolomics, and proteomics—researchers can now gain a comprehensive understanding of pigment biosynthesis and optimize tomato color for both consumer appeal and agricultural success.
A study published in Plant Physiology and Biochemistry in May 2024 revealed that fasciclin-like arabinogalactan proteins (FLAs) play an inhibitory role in carotenoid synthesis during tomato fruit ripening, as shown through integrated transcriptomics and metabolomics analysis. The researchers conducted carotenoid-targeted metabolomics and transcriptomics analyses at MetwareBio across six developmental stages of tomato fruit. Using Weighted Gene Co-expression Network Analysis (WGCNA), they identified several SlFLA genes that were significantly negatively correlated with carotenoid content and genes involved in carotenoid synthesis. Silencing SlFLA13 led to notable phenotypic changes, including earlier fruit ripening and increased carotenoid accumulation, by regulating the expression of carotenoid synthesis-related genes. This study not only highlights the evolutionary characteristics of the FLA gene family in tomatoes but also elucidates their functional role in fruit ripening. These findings expand the scope of research into the biological functions of the FLA family and lay a foundation for future studies on carotenoid synthesis in tomato fruits.
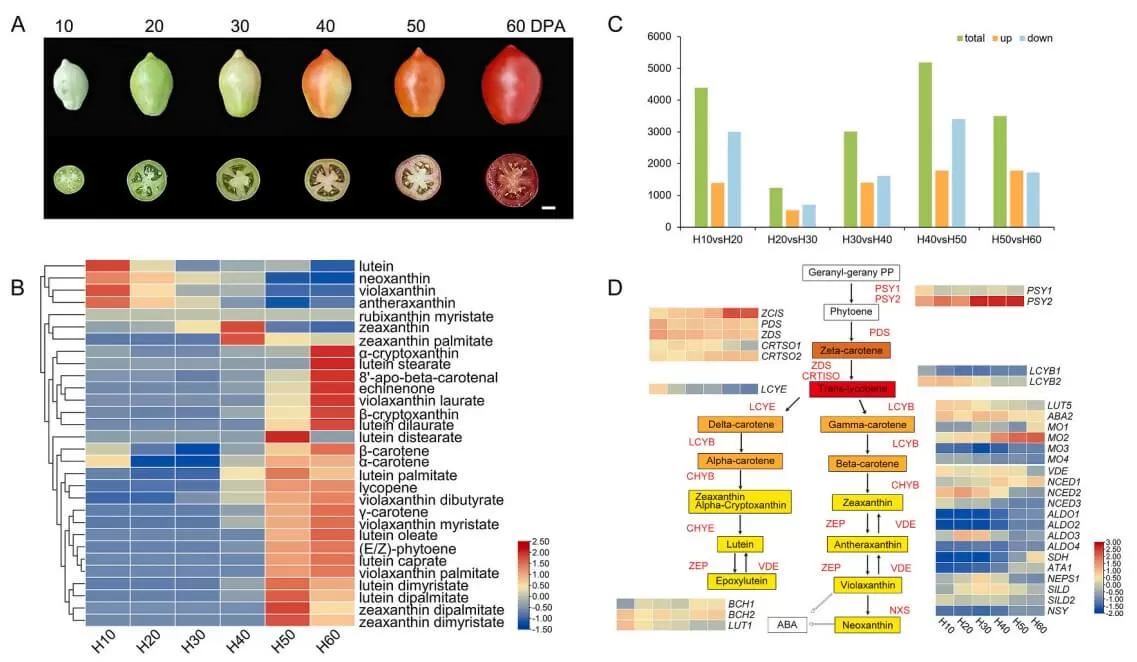
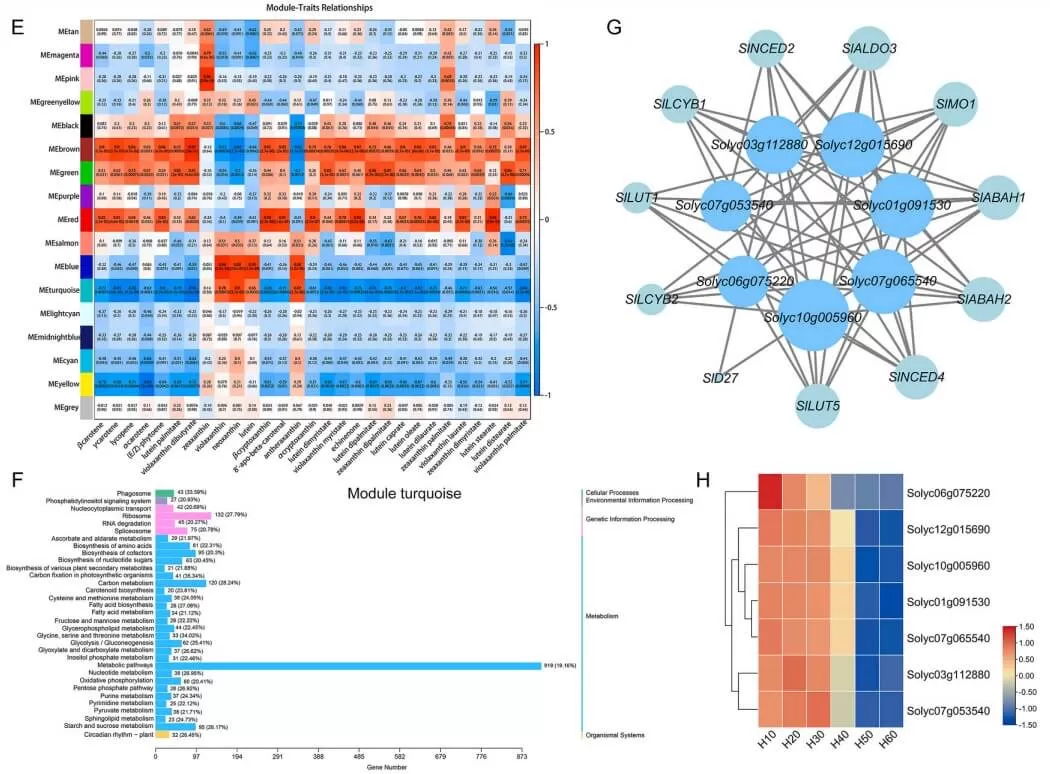
Carotenoid metabolomics and transcriptomics analysis of six development stages tomato fruit (Hu et al., 2024)
Advancing Tomato Stress Tolerance Research with Multi-Omics
Stress tolerance is crucial for plants, especially tomatoes, which are highly susceptible to environmental challenges like drought, heat, and salinity. As a major global crop, improving stress resilience in tomatoes is key to ensuring stable yields and maintaining quality under fluctuating conditions. Traditional breeding methods have limitations in addressing these complexities, which is where multi-omics comes in. By integrating two or more layers of omics data, multi-omics provides a comprehensive understanding of the genetic and metabolic networks driving stress responses. This approach not only uncovers key regulatory pathways but also supports the development of more resilient tomato varieties, critical for sustainable agriculture and food security.
A study published in Plant Physiology in March 2024 revealed that the protein kinase CPK27 interacts with and phosphorylates the tonoplast sugar transporter TST2, facilitating the accumulation of intercellular soluble sugars during drought stress and enhancing drought tolerance in tomatoes. This breakthrough was achieved through an integrated approach combining proteomics, phosphoproteomics, and metabolomics.
The researchers discovered that CPK27 positively regulates drought tolerance in tomatoes. To uncover the detailed regulatory mechanisms, they first conducted quantitative proteomics and phosphoproteomics analyses on tomato leaves from wild-type (WT) plants and cpk27 mutants under both normal and drought conditions. The proteomic analysis identified 122 drought-induced proteins that are dependent on CPK27, with Gene Ontology (GO) enrichment revealing a strong link between CPK27-mediated drought tolerance and carbohydrate metabolism. The phosphoproteomics analysis identified 285 CPK27-associated phosphoproteins, with GO enrichment showing a significant association with monosaccharide and hexose catabolic processes.
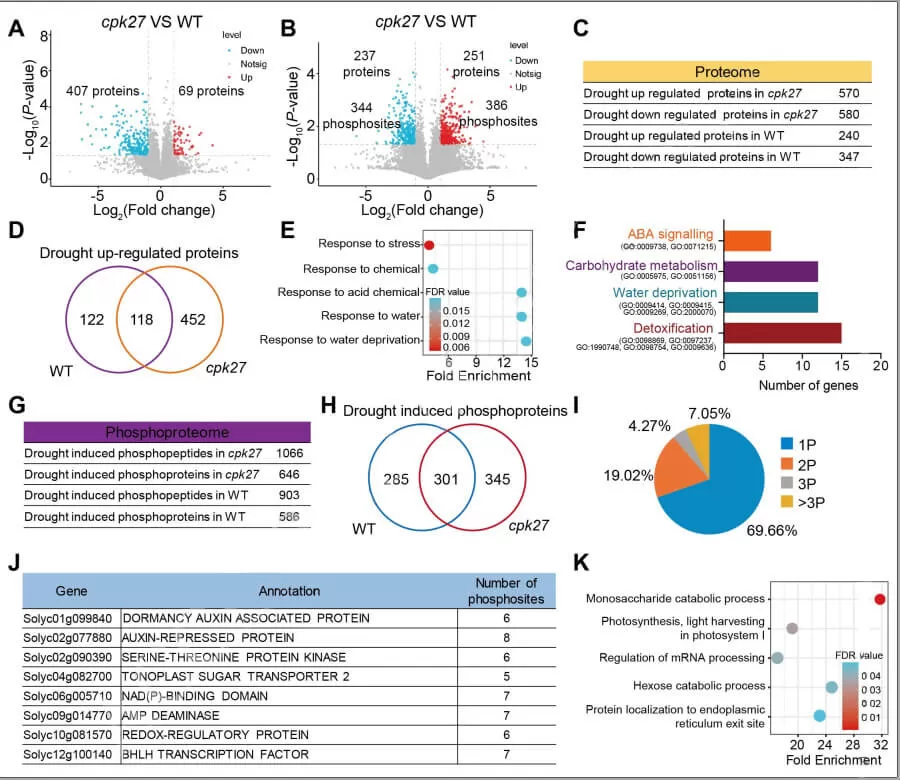
Proteomic and phosphoproteomic profiling of cpk27 and WT plants before and after drought stress (Zhu et al., 2024)
Next, the researchers used sugar-targeted metabolomics, provided by MetwareBio, to investigate the impact of drought on soluble sugar accumulation in cpk27 mutants. While drought treatment significantly increased 19 out of 25 tested soluble sugars, 15 sugars—including trisaccharides (raffinose), disaccharides (maltose, sucrose, cellobiose, trehalose, phenylglucoside), and monosaccharides (glucose, D-fructose, xylose)—showed markedly suppressed levels in cpk27 mutants. These results suggest that CPK27 regulates sugar metabolism to modulate the response to drought stress in tomatoes. Based on this, the researchers identified TST2, a tonoplast sugar transporter, as a direct CPK27-interacting protein through yeast two-hybrid (Y2H) assays. Ultimately, they constructed a complete regulatory network involving TST2 and CPK27 that governs drought stress responses in tomatoes.
These findings extend the toolbox of potential interventions for enhancing plant drought stress tolerance and provide a target to improve drought tolerance by manipulating CPK27-mediated soluble sugar accumulation for rendering drought tolerance in a changing climate.
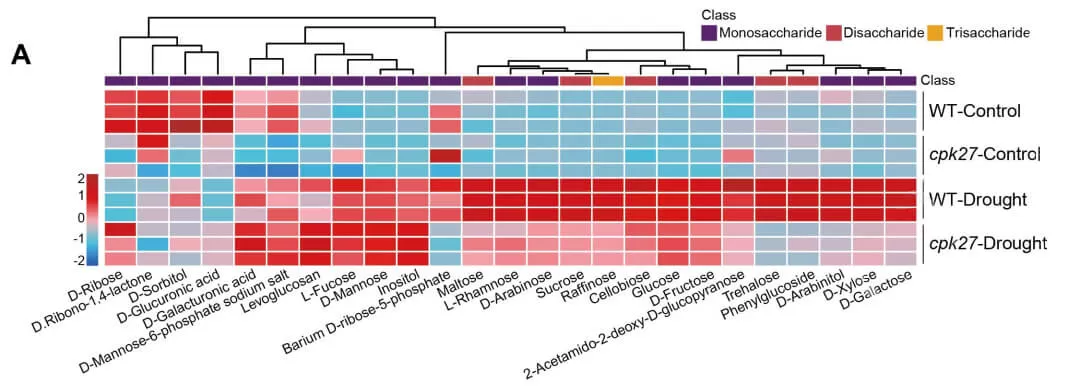
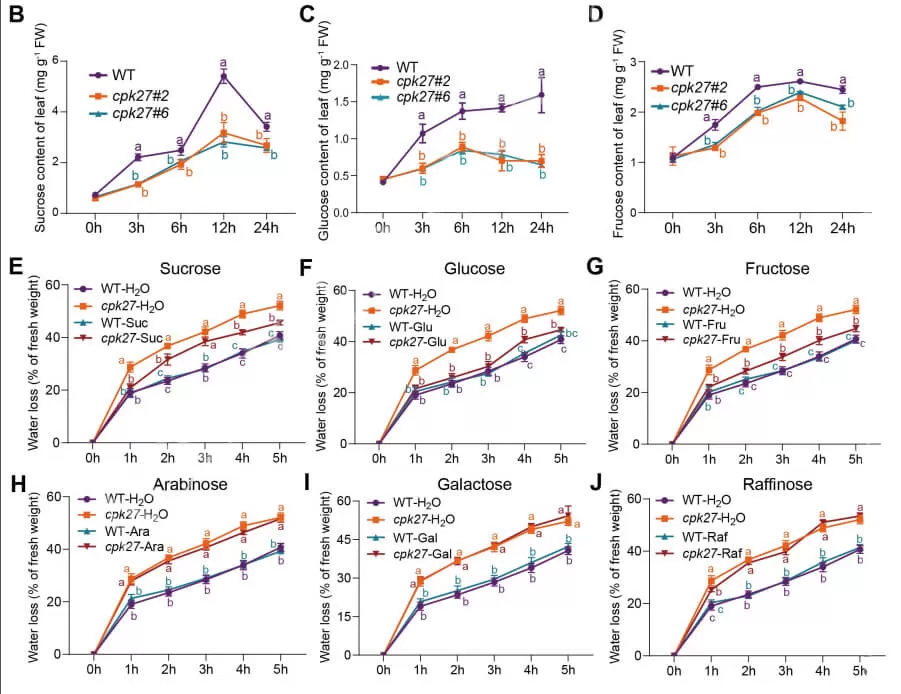
Drought-induced sugar accumulation was impaired in cpk27 mutants (Zhu et al., 2024)
Revealing Tomato Development Research with Multi-Omics
Fruit development is a critical phase in the life cycle of plants, directly influencing yield, quality, and crop productivity. In tomatoes, understanding the complex molecular and genetic factors that regulate fruit growth and maturation is essential for improving agricultural practices and ensuring consistent harvests. One of the major challenges in tomato production is premature fruit drop, a condition that can significantly reduce yields. This phenomenon, often triggered by environmental stress or hormonal imbalances, results in the early shedding of developing fruit before it can fully mature. By applying multi-omics—integrating transcriptomics and/or proteomics, and metabolomics—researchers can gain a comprehensive understanding of the genetic and molecular networks that control tomato fruit development, offering new strategies to enhance fruit set, growth, and overall crop stability, and paving the way for more resilient tomato varieties.
A study published in Journal of Integrative Plant Biology in February 2024 finds that the SlBEL11 –SlMYB111 module modulates flavonoid biosynthesis to fine-tune auxin efflux from fruits and thus maintain an auxin response gradient in the pedicel, thereby preventing premature fruit drop in tomato, through combined analysis of transcriptomics and metabolomics.
The researchers discovered that knockdown of SlBEL11 induces premature fruit drop in tomatoes. To investigate this, they first performed RNA sequencing (RNA-seq) on the fruit abscission zone (FAZ) of SlBEL11-RNAi and wild-type (WT) lines at the mature green (MG) stage. Their analysis revealed a significant down-regulation of genes involved in auxin signaling and flavonoid biosynthesis in the SlBEL11-RNAi lines. These findings suggest that SlBEL11 may regulate the expression of genes related to flavonoid biosynthesis, which in turn suppresses auxin transport and signaling, underscoring its critical role in tomato fruit development.
_1735887028_WNo_884d957.webp)
SlBEL11 regulates genes involved in flavonoid biosynthesis (Dong et al., 2024)
Next, the researchers conducted widely targeted metabolomics and flavonoid-targeted metabolomics at MetwareBio on the FAZ of SlBEL11-RNAi and WT lines at the MG stage. They confirmed that flavonoid levels were significantly lower in the SlBEL11-RNAi lines, with quercetin showing the greatest decrease among all detected flavonoids. To further explore this, spatial metabolomics using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry imaging was performed. This analysis confirmed that quercetin specifically accumulated in WT FAZs during both stages of fruit development, while significantly lower quercetin signals were observed in the FAZ of SlBEL11-RNAi lines. These findings suggest that flavonoids play a role in SlBEL11-regulated fruit abscission in tomatoes.
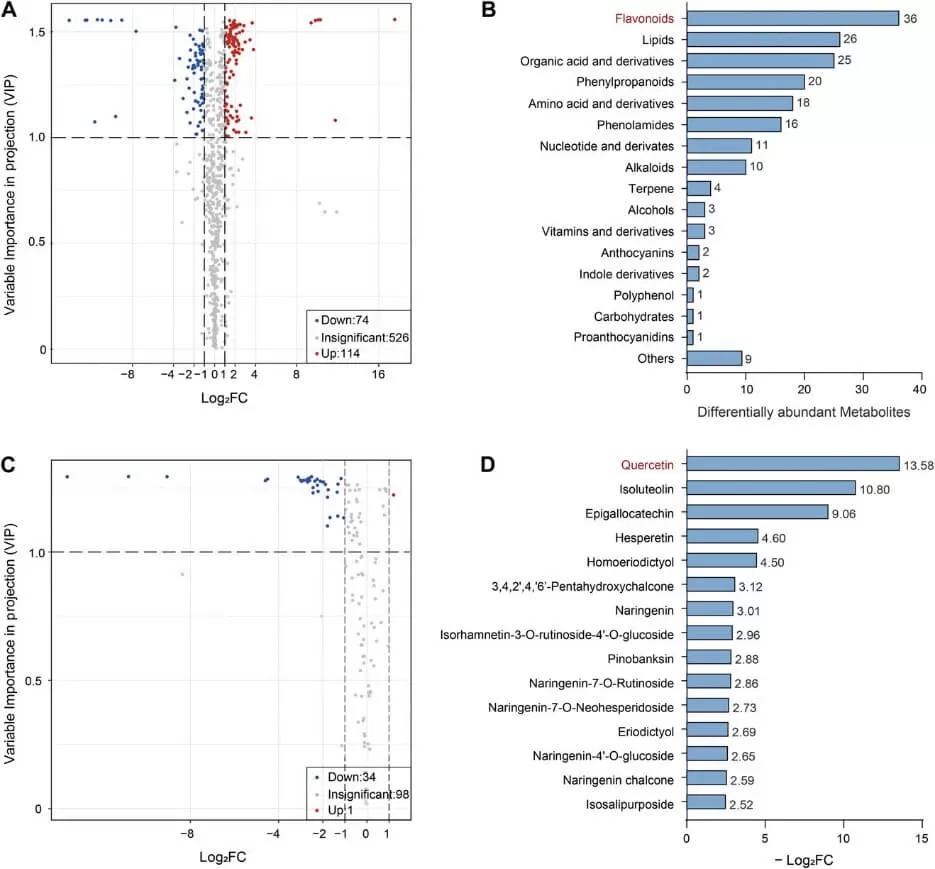
Widely targeted and targeted metabolomics of SlBEL11-RNAi plants (Dong et al., 2024)
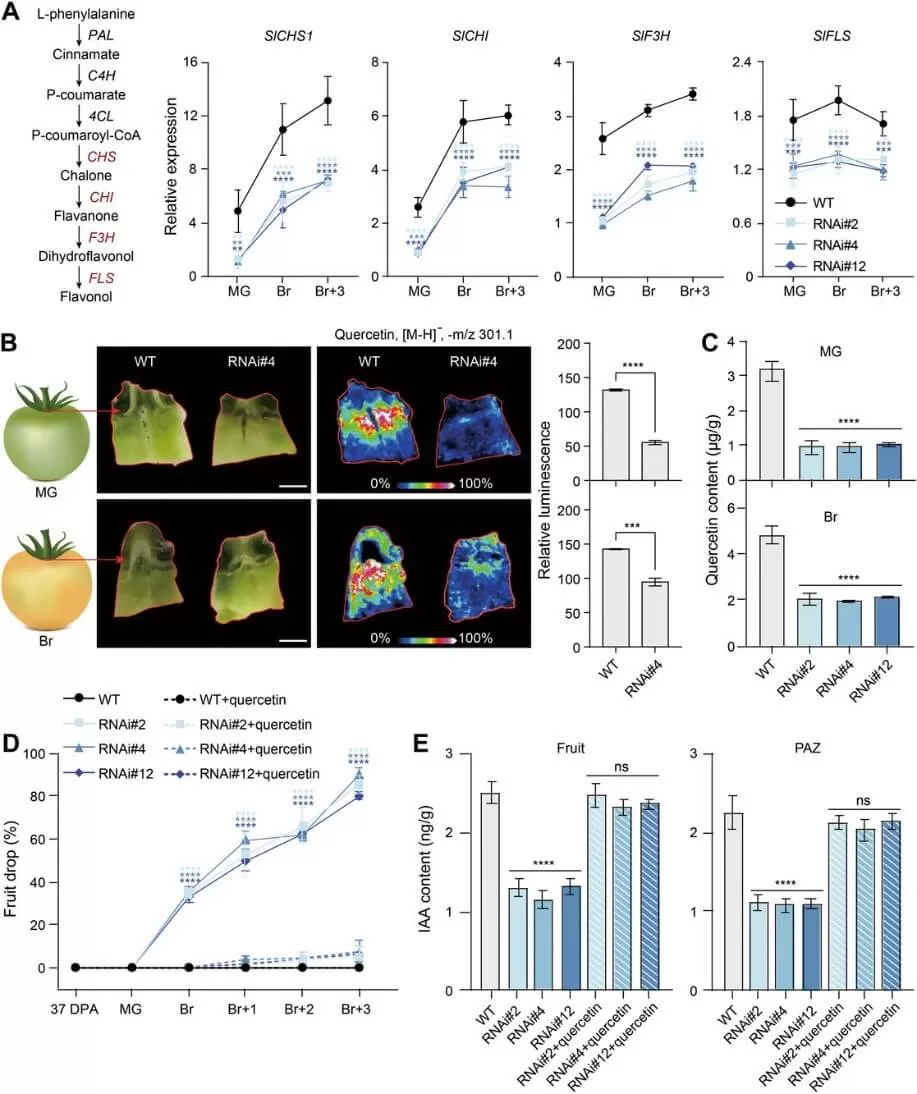
Quercetin treatment suppresses the premature fruit drop of SlBEL11-RNAi plants (Dong et al., 2024)
Building on these results, the researchers revealed the regulatory mechanism: SlBEL11 induces the expression of SlMYB111, a key regulator of core flavonoid biosynthesis genes, promoting flavonoid accumulation in the FAZ. The accumulated flavonoids inhibit auxin transport, maintaining an optimal auxin level in the FAZ. This regulation ensures proper auxin transport across the pedicel abscission zone (PAZ), preventing premature fruit abscission. These findings provide new insights into the molecular mechanisms regulating fruit abscission in tomatoes.
Transforming Plant Science Through MetWareBio’s Multi-Omics
These practical applications of multi-omics in tomato research reveal the vast potential of this approach in plant science. By harnessing the power of integrated omics technologies, researchers can unlock new layers of complexity in plant biology, revealing insights that were previously out of reach. At MetWareBio, we are proud to be at the forefront of this revolution, offering comprehensive and cutting-edge multi-omics services including transcritptomics, proteomics and metabolomics that empower scientists to explore plant metabolism, protein functions, and spatial dynamics with unprecedented depth.
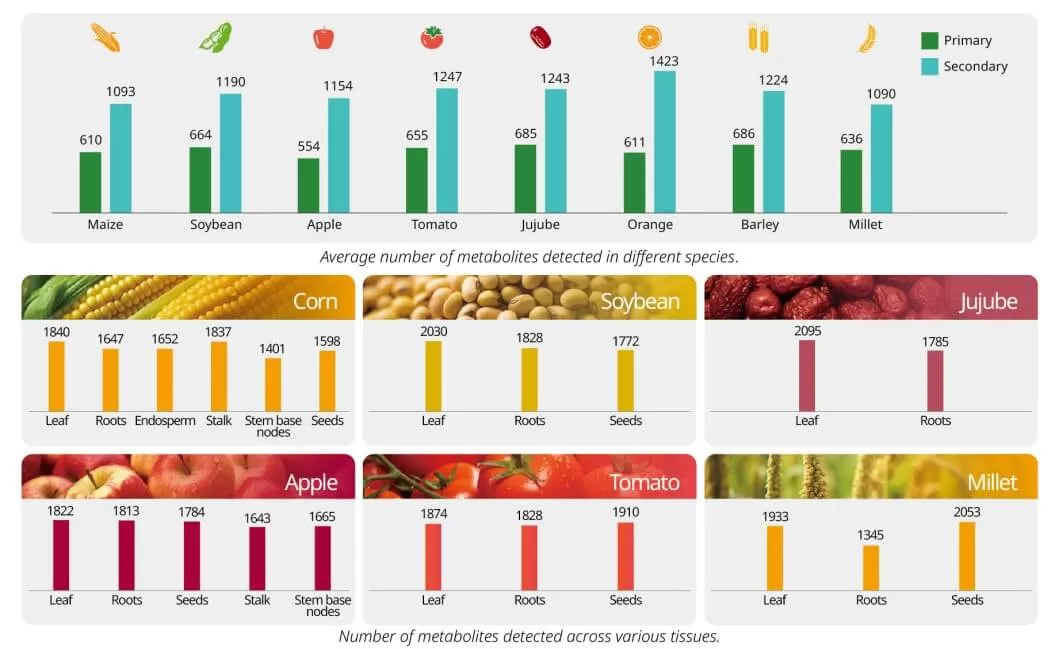
Beyond classic targeted metabolomics, such as carotenoid, anthocyanidin, and plant hormone analysis, our plant widely-targeted metabolomics service is an invaluable tool for revealing the complex and comprehensive metabolic profiles of plants. With access to a comprehensive database containing over 35,000 compounds, we can identify a vast range of metabolites, including key classes such as flavonoids, phenolic acids, alkaloids, terpenoids, and more. Whether you're exploring plant secondary metabolites or primary metabolic pathways, our targeted approach enables the identification of 1,500+ metabolites on average from a variety of plant species and tissues. This wide-ranging service is perfect for applications in plant breeding, stress response research, and the discovery of novel compounds with potential agricultural or pharmaceutical applications.
|
Widely-targeted metabolomics Database in Plants (MWDB V4.5) |
||
|
Types |
Number |
Representative compounds |
|
Flavonoids |
5000+ |
Rutin, Phloretin, Phelligrin A, Hesperetin, Licorice glycoside A, Pelargonidin-3-O-glucoside, Ginkgetin, Formononetin, Theaflavin |
|
Phenolic acids |
2500+ |
Chlorogenic acid, Momordicoside A, Oleuropein, Salvianolic acid A, Tatariside A, Veratric acid, Salidroside, Parishin B, Magnoloside A, Vanillic acid |
|
Alkaloids |
8000+ |
α-Solanine, Verticine, Nuciferine, Stachydrine, Matrine, Camptothecin, Arecoline, DIMBOA, Avenanthramide A, Lycorenine |
|
Terpenoids |
8500+ |
Artemisinine, Genipin, Paclitaxel, Wilforlide A, Protopanaxdiol, Saikosaponin A, Cucurbitacin B, Crocin I, Cyclocarioside Ⅰ, Ecliptasaponin A |
|
Quinones |
800+ |
Emodin, Obtusin, Lapachone, Shikonin, Tectograndone, Morindaparvin A, Aloesin, 5-Hydroxydigitolutein, Trijuganone A |
|
Steroid |
1500+ |
Asparagoside C, Polyphyllin I, Timosaponin A-III, Gracillin, Sarsasapogenin, Tigogenin, Digitonin, Oleandrin |
|
Tannins |
300+ |
Ellagic acid, Gemin D, Casuariin, Punicalin, Chebulagic acid, 1,3,6-Tri-O-galloylglucose, Chebulanin, Tellimagrandin I |
|
Ligans |
1200+ |
Honokiol, Syringaresinol, Arctigenin, Pinoresinol, Schisanhenol, Sesamin, Chestnutlignansoide, Trachelogenin, Fargesin, Isolariciresinol |
|
Glucosinolates |
200+ |
Sulforaphane, Gluconasturtiin, Sinalbin, Glucocheirolin, Glucoraphanin, 4-Hydroxy-3-indolylmethyl glucosinolate, Sinigrin, 4-Methylsulfinyl-3-Butenyl Glucosinolate |
|
Coumarins |
800+ |
Umbelliprenin, Psoralen, Glycycoumarin, Xanthotoxol, Scopolin, Bengenin, Bergapten, Decursinol, Dihydrocoumarin |
|
Organic acids |
300+ |
Succinic acid, Malic acid, Citric Acid, Quinic Acid, Abscisic acid, Tartaric acid, Shikimic acid, Aconitic Acid, Salicylic acid, Cinnamic acid, Maleic acid |
|
Vitamins |
50+ |
Vitamin C, Vitamin B2, Vitamin A1, Vitamin U, Ginkgotoxin, Nicotinic acid, Nicotinamide, Retinol, Vitamin D3, Tocotrienol |
|
Amino acids and derivatives |
670+ |
Tryptophan, Theanine, Beauvericin, Dencichin, Heterophyllin, Saccharopine, Alliin, Dopa, S-Adenosylmethionine, γ-Glu-Cys |
|
Nucleotides and derivatives |
150+ |
Adenine, Cytosine, Thymine, Inosine, Eritadenine, Xanthosine, Cordycepin, Sepiapterin, Adenosine 5'-monophosphate |
|
Saccharides and Alcohols |
340+ |
Glucose, Fructose, Sucrose, Fucose, Xylitol, Rhamnose, Maltose, Raffinose, Allitol, Mannitol |
|
Lipids |
700+ |
Linolenic acid, 4-Hydroxysphinganine, Lauric acid, Myristic Acid, Palmitic acid, Arachidonic Acid, Stearic Acid |
|
Others |
4200+ |
Aflatoxin B1, Secoxyloganin, Kavain, Terreic acid, Mansonone E, Litchiol A, Myricanol, Safranal, Bruceine A, Gambogic acid |
|
Total |
35000+ |
|
In addition to traditional metabolomics, MetWareBio’s spatial metabolomics service offers high-resolution imaging (up to 5 µm), enabling detailed mapping of metabolite distribution within plant tissues. This tool reveals metabolite localization at the cellular level, providing valuable insights into plant physiology, stress responses, and tissue-specific metabolic processes. It is particularly useful for studying plant development, pathogen defense, and precision agriculture.
Moreover, our proteomics services complement our metabolomics capabilities by providing detailed insights into the proteome and post-translational modifications. Through DIA quantitative proteomics, we provide high-throughput, reproducible analysis of protein expression across various plant species, allowing for the identification and quantification of thousands of proteins. For studies focused on protein function and regulation, our phosphoproteomics service provides in-depth analysis of protein phosphorylation events, which are crucial for understanding signal transduction pathways and cellular responses to various stimuli.
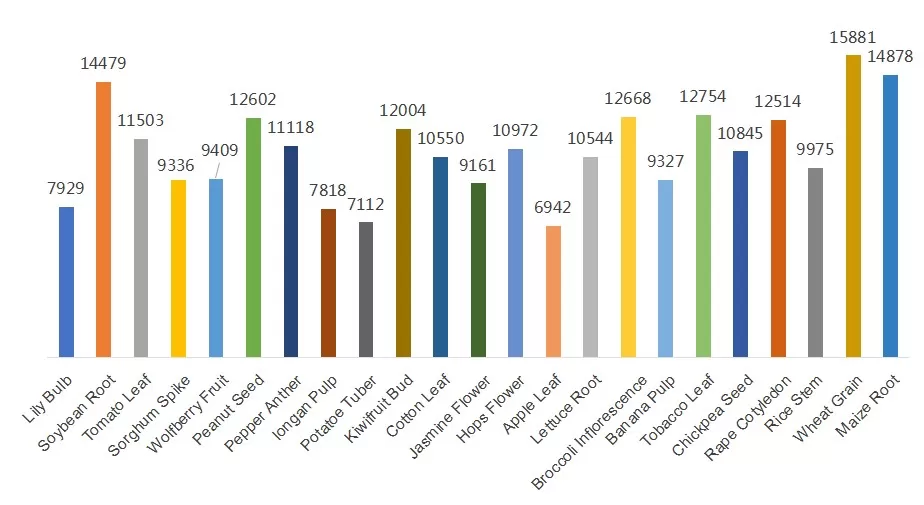
Number of proteins detected in different plant samples using DIA quantitative proteomics
At MetWareBio, we combine our multi-omics expertise, cutting-edge technologies, and in-depth knowledge of plant biology to offer the most comprehensive and reliable services for advancing your research. Whether you're investigating plant metabolism, proteomics, or spatial dynamics, our multi-omics platforms are designed to provide actionable insights that will propel your plant science research to new heights. Let us be your partner in pioneering breakthroughs that can transform agriculture, biotechnology, and environmental sustainability.
Reference
Hu J, Wang J, Muhammad T, et al. Functional analysis of fasciclin-like arabinogalactan in carotenoid synthesis during tomato fruit ripening. Plant Physiol Biochem. 2024;210:108589. doi:10.1016/j.plaphy.2024.108589
Zhu C, Jing B, Lin T, et al. Phosphorylation of sugar transporter TST2 by protein kinase CPK27 enhances drought tolerance in tomato. Plant Physiol. 2024;195(2):1005-1024. doi:10.1093/plphys/kiae124
Dong X, Liu X, Cheng L, et al. SlBEL11 regulates flavonoid biosynthesis, thus fine-tuning auxin efflux to prevent premature fruit drop in tomato. J Integr Plant Biol. 2024;66(4):749-770. doi:10.1111/jipb.13627
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


