Why You Shouldn't Skip Breakfast: Multi-Omics Insights into Nutrient Absorption Mechanisms in the Small Intestine
In today's fast-paced world, many of us often skip breakfast, believing it to be a harmless habit. However, emerging research suggests that this seemingly innocuous choice can have profound implications for our health. The small intestine plays a pivotal role in nutrient supply and absorption, serving as the body's primary site for processing the food we consume. By leveraging multi-omics approaches, scientists are uncovering intricate details about how our dietary patterns—especially the omission of breakfast—affect the mechanisms of nutrient absorption. In this blog, we will explore the significant insights gained from multi-omics research, revealing the potential risks associated with skipping breakfast and highlighting the essential functions of the small intestine in maintaining overall health.
Anatomy and Function of the Small Intestine
The small intestine is a vital organ in the digestive system, responsible for the majority of digestion and nutrient absorption. Stretching from the stomach to the large intestine, it is a long, narrow, coiled tube situated in the central and lower abdominal cavity. Measuring approximately 6.7 to 7.6 meters (22 to 25 feet), its intricate folds create a large surface area that enhances digestion and absorption efficiency.
The small intestine consists of three specialized sections—the duodenum, jejunum, and ileum—each with distinct functions and a cellular structure finely tuned to optimize nutrient uptake.
1. Duodenum: The first part of the small intestine, where partially digested food (chyme) from the stomach mixes with digestive enzymes from the pancreas and bile from the liver. This section is primarily responsible for breaking down food into absorbable components.
2. Jejunum: This middle section is the main site of nutrient absorption. The surface is covered with tiny finger-like projections called villi and microvilli, forming a brush border that greatly increases surface area and allows for efficient nutrient uptake.
3. Ileum: The final segment, the ileum, continues to absorb nutrients, particularly vitamins like B12 and bile acids, before passing any undigested material to the large intestine.
Cell Composition and Structure of the Small Intestine
The wall of the small intestine has multiple layers, each with specialized cells and structures designed to facilitate digestion and absorption:
1. Epithelial Layer:
- Enterocytes are the most abundant cells, primarily responsible for nutrient absorption. Their microvilli form a brush border that not only increases surface area but also contains enzymes to further break down nutrients.
- Goblet Cells secrete mucus to lubricate and protect the intestinal walls, facilitating smooth movement and shielding the lining from digestive enzymes.
- Paneth Cells, located at the base of intestinal crypts, secrete antimicrobial peptides (such as defensins and lysozymes), providing defense against harmful bacteria.
- Enteroendocrine Cells release hormones that regulate digestion and nutrient uptake, influencing enzyme and bile secretion as well as gut motility.
2. Lamina Propria: This connective tissue layer beneath the epithelium contains blood vessels, lymphatics, and immune cells, which support nutrient transport and immune protection.
3. Muscularis Mucosae: A thin muscle layer that maintains the structural integrity of the mucosa and helps form the folds and villi, increasing the absorptive surface area.
4. Submucosa: A thicker layer of connective tissue containing larger blood vessels, lymphatics, and nerves, including the Meissner's plexus, which regulates local blood flow, secretion, and muscle movement.
5. Muscularis Externa: This smooth muscle layer, consisting of inner circular and outer longitudinal muscle layers, facilitates peristalsis (the wave-like contractions moving food through the intestine). The Auerbach's plexus within this layer regulates muscle contractions for effective motility.
6. Serosa (Outer Layer): This thin outer layer consists of connective tissue covered by mesothelial cells, forming part of the peritoneum, which reduces friction with surrounding organs.
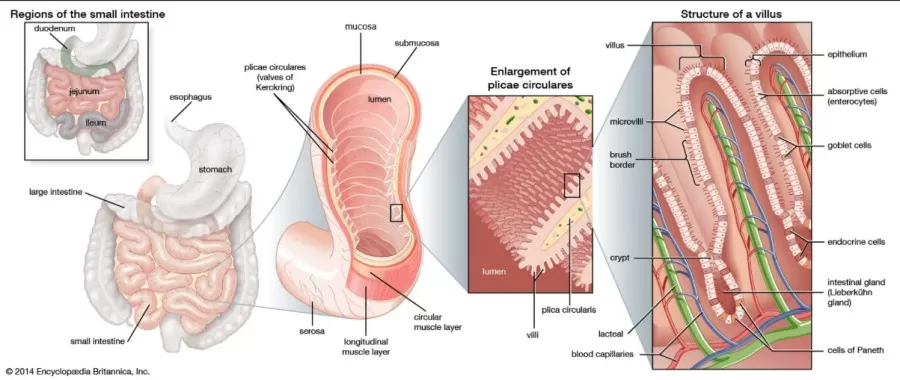
Image come from website https://www.britannica.com/science/small-intestine
The specialized layers and cell types within the small intestine enable it to efficiently perform digestion and nutrient absorption while also providing structural support, immune defense, and smooth food passage. Through its intricate structure and highly specialized cellular composition, the small intestine is indispensable for extracting nutrients that fuel bodily functions.
Multi-Omics Reveal Nutrient Supply and Absorption Mechanisms in the Small Intestine
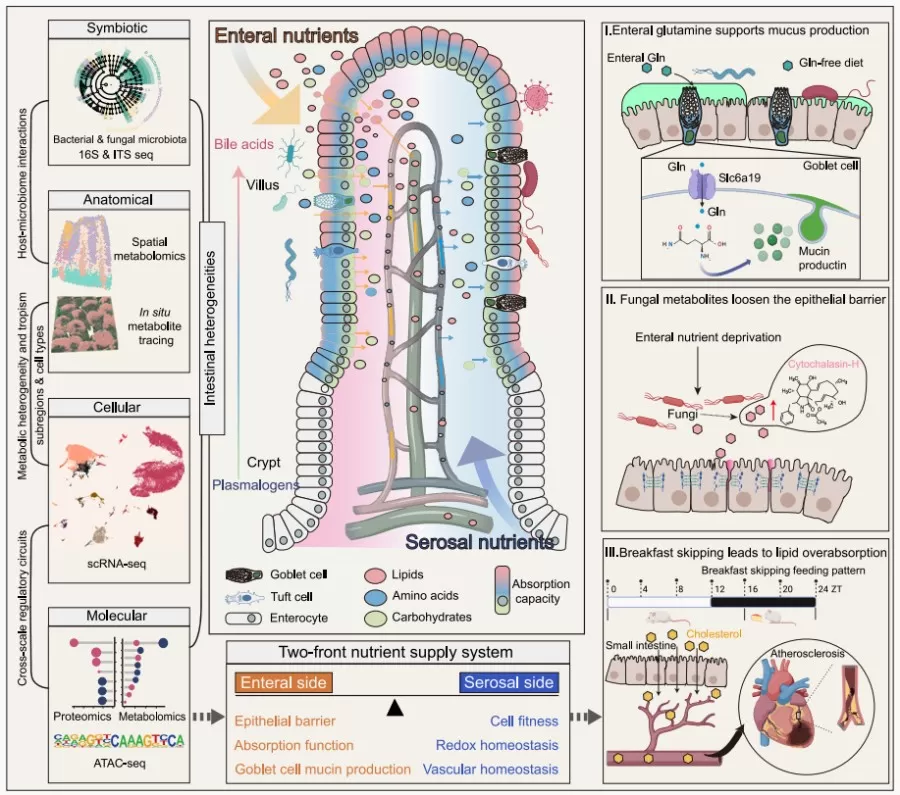
The small intestine is a complex multicellular system responsible for nutrient absorption, hormone secretion, microbial interaction, and immune defense. With a high rate of cell renewal and spatiotemporal heterogeneity, the small intestine has unique energy demands, requiring an efficient nutrient supply system to maintain its functions. The small intestine has a unique dual supply system that provides nutrients from both the intestinal lumen and the peritoneal side, resulting in distinct physiological impacts on specific regions and cell types. The contrasting effects of orally administered versus intravenously injected glucose underscore the influence of nutrient entry routes on intestinal and systemic physiological responses. However, the relative contributions of luminal and blood-derived nutrients to small intestinal physiology and their underlying mechanisms remain underexplored.
Recently, a study published in the leading journal Cell, titled “A two-front nutrient supply environment fuels small intestinal physiology through differential regulation of nutrient absorption and host defense,” employed multi-omics technologies (metabolomics, proteomics, transcriptomics, and microbiomics) to deeply investigate the characteristics and dynamics of the small intestine’s dual nutrient supply system. The study highlights the spatiotemporal differences in nutrient absorption arising from different nutrient pathways and types and their regulatory effects on host defense mechanisms (such as mucosal immunity and barrier function) and nutrient absorption (including lipid and overall metabolism). Additionally, the research reveals how disruptions in nutrient intake, such as skipping breakfast, can lead to excessive lipid absorption, thereby contributing to the progression of cardiovascular disease.
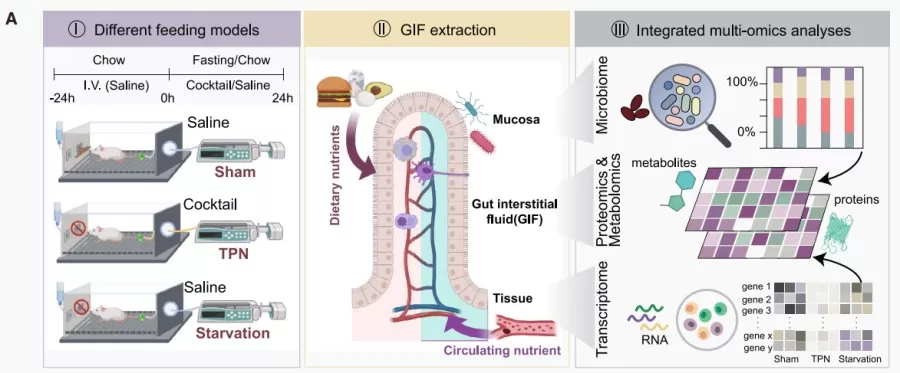
To investigate the effects of luminal and extraluminal nutrient supply on the body, researchers designed three experimental groups of mice: a control group (ad libitum feeding with intravenous saline injection), a parenteral nutrition group (feed-deprived with intravenous parenteral nutrition solution), and a fasting group (feed-deprived with intravenous saline injection). After a 24-hour acclimation period, small intestinal interstitial fluid (GIF) was collected from all groups, followed by metabolomic, proteomic, transcriptomic, and microbiomic analyses to examine differences in nutrient supply across feeding conditions.
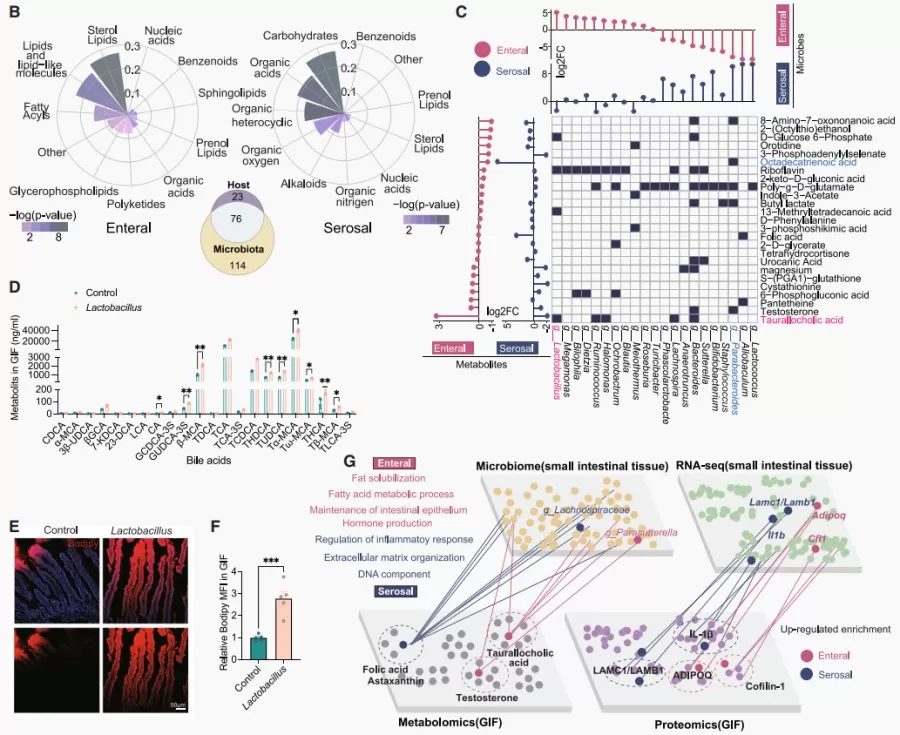
Results indicated that the intestinal nutrient supply primarily facilitated lipid metabolism, while the serum nutrient supply was more associated with carbohydrate and organic acid metabolism. Additionally, microbial metabolites were found to have a significant impact on the intestinal environment. In-depth analysis revealed that luminal nutrient supply supports lipid absorption and barrier function, whereas the serum nutrient supply promotes tissue structure and immune regulation, highlighting the complex nutrient supply characteristics of the small intestine.
The research further explored the spatiotemporal characteristics of nutrient absorption in the enteral-serosal supply system. Results showed that nutrient absorption on the luminal side of the intestine displayed marked regional specificity: glucose was absorbed uniformly along the villi, cholesterol predominantly at the villus tips, and glutamine was enriched in goblet and tuft cells. In contrast, nutrient absorption on the serosal side showed no distinct region or cell-type specificity within the villus structure. Using spatial metabolomics combined with tissue staining and single-cell RNA sequencing, the study examined metabolic heterogeneity and functional specialization within the villi, revealing significant metabolic specificity in the small intestine under different nutrient supply modes. Enteral supply had a more pronounced impact on various intestinal cells, particularly enterocytes, by activating functions related to nutrient absorption and oxidative metabolism, whereas serosal supply supported cell proliferation. Overall, luminal nutrition had a more critical influence on small intestinal physiology.
The study also investigated the adaptive absorption mechanisms of enterocytes under nutrient deficiency, focusing on the effects of a “breakfast skipping” (BSF) model on intestinal absorption functions. Findings indicated that irregular breakfast skipping could lead to long-term alterations in lipid absorption in the small intestine through chromatin remodeling, posing potential health risks. Mechanistically, the BSF model significantly increased the expression and endocytosis of the cholesterol transporter Npc1l1 in enterocytes, thereby enhancing cholesterol absorption. Under a high-cholesterol diet (HCD), BSF raised serum levels of total cholesterol, triglycerides, and low-density lipoprotein cholesterol (LDL-C), while reducing cholesterol excretion in feces. Furthermore, BSF significantly increased atherosclerotic lesion area and lipid and collagen deposition within lesions, suggesting that BSF may exacerbate cardiovascular disease risk by promoting intestinal cholesterol absorption.
In conclusion, this study employed multi-omics approaches—including metabolomics, proteomics, transcriptomics, and microbiomics—to systematically construct a comprehensive overview of the small intestine under a dual nutrient supply model. It uncovered the diverse regulatory mechanisms by which different nutrient pathways and types influence the intestinal absorption process, including mucus production, barrier integrity, and lipid absorption. The research elucidated how imbalances in luminal nutrient supply (e.g., skipping breakfast) can lead to excessive lipid absorption in the small intestine, thereby accelerating cardiovascular disease progression. These findings deepen our understanding of the regulatory mechanisms governing the small intestine under varying nutrient supply conditions.
Partner with MetwareBio for Cutting-Edge Metabolomics and Multi-Omics Research
MetwareBio provided untargeted metabolomics services for this research, revealing the heterogeneity of nutrient absorption in the small intestine under various nutrient supply modes. Our high-quality metabolomics data significantly contributed to understanding distinct metabolic pathways and absorption mechanisms within the intestinal environment.
At MetwareBio, we are dedicated to advancing innovation in metabolomics and multiomics technologies, offering comprehensive services in metabolomics, lipidomics, proteomics, and more. Our untargeted metabolomics platform enables in-depth exploration of complex biological processes, utilizing robust analytical pipelines and extensive compound libraries for precise metabolite identification and quantification. Through our cutting-edge instrumentation and optimized workflows, we empower researchers to gain high-resolution insights into metabolic pathways, providing unparalleled support in studies related to nutrition, health, and disease mechanisms.
With MetwareBio’s integrated multiomics approach, researchers obtain a holistic view of biological interactions across metabolomic, proteomic, and other omics layers, facilitating the identification of key molecular networks and regulatory mechanisms. Our services are a powerful resource for advancing scientific discovery and fostering breakthroughs in areas such as precision nutrition, metabolic health, and disease progression.
Choose MetwareBio for your metabolomics and multiomics needs and unlock the potential of your research with our state-of-the-art services.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


