Beyond Single-Omics: A Guide to Multi-Omics Association Analysis
In today’s advanced research landscape, relying on a single-omics approach—whether transcriptomics, proteomics, metabolomics, or microbiomics—is no longer sufficient to fully grasp the complexity of biological systems. Multiomics association analysis is becoming increasingly popular as scientists seek to integrate multiple omics layers to reveal deeper biological insights. By combining data from transcriptomics, proteomics, metabolomics, and microbiomics, researchers can uncover how these interconnected systems shape phenotypes and responses to environmental factors. Multiomics association analysis is revolutionizing both human health research and agricultural innovation, providing a more holistic view of biological processes. In this blog, we will introduce the principles and approach of different multi omics analyses, exploring how they work together to unlock complex biological networks and drive innovation in various fields.
What Does Single-Omics Research Involve?
Before diving into what multiomics association analysis entails, let’s first explore the key research areas of popular single-omics approaches, such as transcriptomics, proteomics, and metabolomics. Each of these omics technologies provides valuable insights into different layers of biological information.
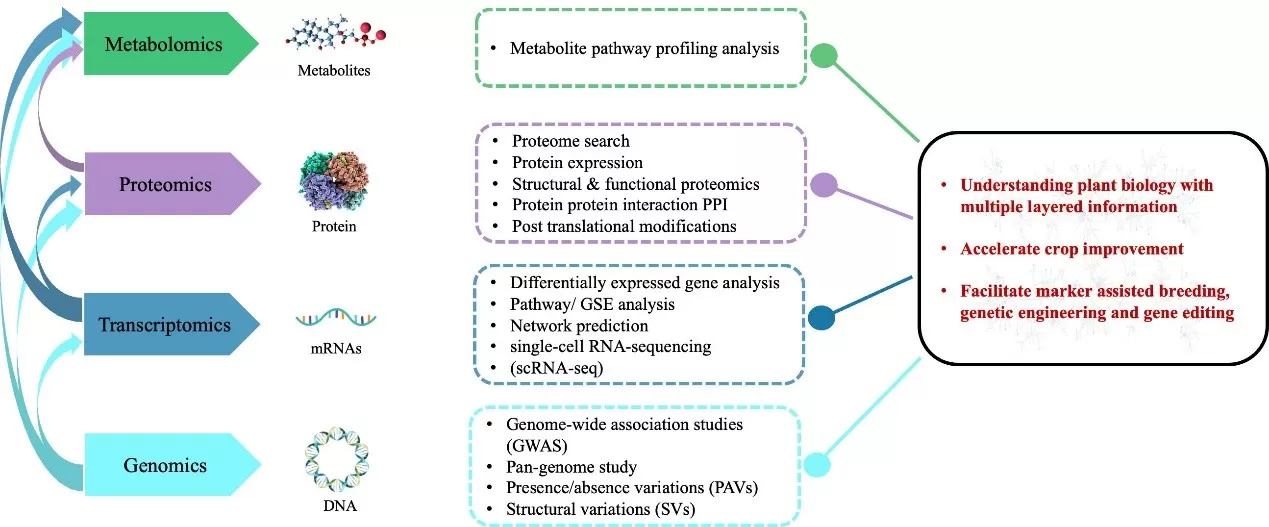
- Transcriptomics: Transcriptomics is the study of the complete set of RNA transcripts produced by a genome at any given time. This includes messenger RNA (mRNA), ribosomal RNA (rRNA), transfer RNA (tRNA), and non-coding RNA (ncRNA). The transcriptome reflects the genes that are actively expressed in a cell or tissue under specific conditions, offering insights into gene expression patterns, regulation, and how cells respond to external stimuli. Transcriptomics is commonly used to understand how gene expression changes in diseases, development, or in response to treatments.
- Proteomics: Proteomics is the large-scale study of proteins, the functional molecules that perform most cellular processes. It involves identifying and quantifying the entire set of proteins (the proteome) in a cell, tissue, or organism at a specific time. Since proteins are dynamic and involved in most cellular activities, proteomics provides insight into biological functions, signaling pathways, protein-protein interactions, and how protein levels and modifications change in response to disease or environmental conditions.
- Metabolomics: Metabolomics is the study of metabolites—small molecules produced during metabolism—within a biological system. The metabolome represents the final downstream products of gene expression and cellular processes, providing a snapshot of an organism’s metabolic state. Metabolomics is widely used to study disease biomarkers, understand metabolic pathways, and explore how environmental factors like diet or drugs affect metabolism.
- Microbiomics: Microbiomics focuses on the study of microbial communities, particularly the collective genomes (microbiomes) of bacteria, viruses, fungi, and other microorganisms residing in specific environments, such as the human gut, soil, or ocean. The microbiome plays crucial roles in host health, digestion, immunity, and even mood regulation. Microbiomics is increasingly used to investigate the influence of the microbiota on human health and disease, as well as its applications in agriculture and environmental science.
What Is Multi-Omics Association Analysis?
Multiomics association analysis refers to the integration and analysis of multiple types of "omics" data to understand complex biological processes, identify molecular signatures, and uncover relationships between different biological layers. By combining data from these different omics layers, researchers can explore how variations in RNA, proteins, and metabolites are interconnected and influence phenotypes, diseases, or treatments. This approach provides a holistic view of biological systems and can reveal intricate regulatory mechanisms, biomarkers for diseases, or potential therapeutic targets that may not be apparent from single-omics studies. The main types of multi omics integration analyses are as follows:
1. Transcriptomics + Proteomics: Exploring Gene Expression and Regulation
This combination aligns with the classical central dogma. Since mRNA levels don’t fully represent gene expression, transcriptomics alone cannot accurately reflect post-transcriptional regulation, protein modifications, or the dynamic balance of synthesis and degradation. Additionally, transcriptomics often yields large datasets, making it difficult to identify the most relevant genes for validation. By integrating transcriptomics and proteomics, we can explore gene expression and regulation more precisely from both post-transcriptional and post-translational perspectives, enabling a more detailed study of key genes.
2. Transcriptomics/Proteomics + Metabolomics: Mapping Metabolic Pathways
Both transcriptomics and proteomics represent gene expression, so the approach for combining transcriptomics with metabolomics or proteomics with metabolomics is nearly identical. Transcriptomics/proteomics provide insights into enzymes, while metabolomics focuses on substrates or products. Together, they form the core of enzymatic reactions, and multiple related reactions create metabolic pathways. By combining these analyses, we can rapidly identify pathways potentially related to phenotypic changes or investigate the differential genes/proteins and metabolites within pathways of interest to validate their role in driving specific phenotypes.
3. 16S rRNA + Metabolomics: Understanding Microbial Influence on Host Metabolism
16S rRNA sequencing analyzes microbial composition and abundance, while metabolomics examines the types and concentrations of host metabolites. This combined approach helps reveal how the microbiota, through its own metabolism, influences host metabolism and phenotypic changes. These analyses are often centered around specific microbiome-related research areas, such as the rhizosphere and phyllosphere microbiota of plants, gut microbiota in animals, or microbial communities in water and soil environments.
How to Perform Multi-Omics Association Analysis?
The key to performing multiomics association analysis lies in effectively integrating data from multiple omics layers. This can be broadly categorized into four approaches: name-based integration, function-based integration, correlation-based integration, and biomarker-based selection, each with its unique methodology and applications.
1. Name-based Integration
This approach is primarily used for transcriptomics and proteomics analysis. It involves identifying shared gene names (or annotations) between the transcriptome and proteome datasets, linking the two layers via the "gene" level. The focus is on comparing the expression levels of the same gene across both omics, exploring cases where genes are upregulated or downregulated in both datasets, or exhibit opposite trends. The results of this analysis can be visually represented using Venn diagrams or nine-quadrant plots to highlight these relationships.
2. Function-based Integration
Function-based integration examines the functional enrichment results from both omics datasets and identifies common KEGG pathways that are significant in both layers, indicating their potential role in molecular mechanisms. For transcriptomics/proteomics combined with metabolomics analysis, this approach allows for detailed pathway analysis, where differential genes/proteins (enzymes) and metabolites (substrates or products) are mapped onto KEGG pathways for deeper exploration. When combining transcriptomics and proteomics, functional enrichment analysis can include Gene Ontology (GO) terms as well. Results can be displayed using Venn diagrams, bubble charts, bar charts, histograms, or KEGG pathway maps.
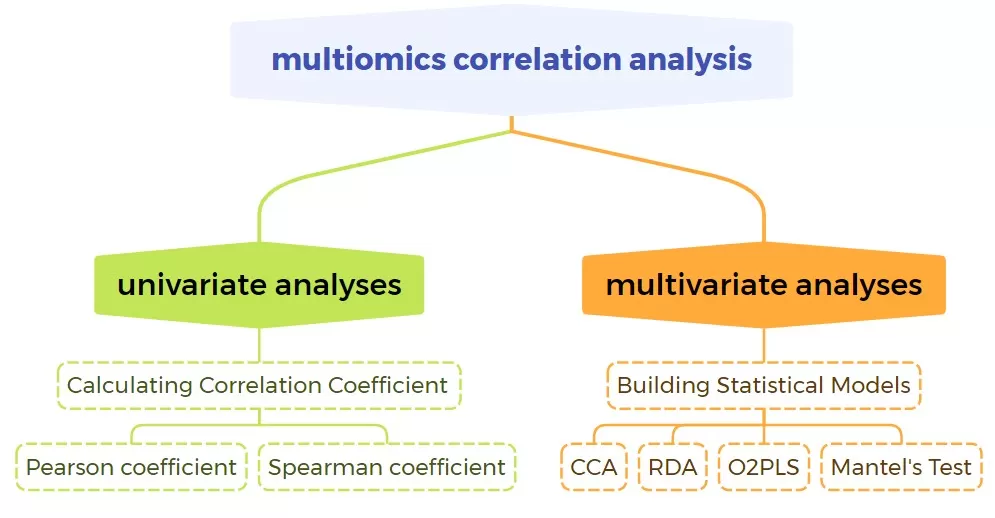
3. Correlation-based Integration
This method uses various biostatistical formulas and algorithms to compute relationships between quantitative data from different omics layers (expression matrices). Correlation-based integration is divided into two main types: univariate and multivariate analyses. Univariate results can be shown through heatmaps, network graphs, scatter plots, and chord diagrams, while multivariate analyses can be presented with methods like Canonical Correlation Analysis (CCA) or Redundancy Analysis (RDA). Unlike function-based integration, correlation-based analysis does not depend on pathway annotations (e.g., limited KEGG annotations), making it highly flexible and providing abundant insights. It is widely used in transcriptomics/proteomics, transcriptomics/proteomics + metabolomics, and metabolomics + 16S microbiomics analyses.
4. Biomarker-based Selection
This specialized approach involves using machine learning models to identify composite biomarker features from multiple omics datasets. These combined features typically have stronger predictive power than individual omics layers. Biomarker-based selection is mainly applied in clinical research and requires large sample sizes. The results are often presented through Receiver Operating Characteristic (ROC) curves to demonstrate the model's predictive performance.
Future Directions of Multi-omics
With the continuous advancement of technology and the continuous expansion of research needs, multi-omics analysis is also developing, showing some new trends and directions.
1. Higher throughput, lower cost
Single-cell multi-omics: Apply multiple omics technologies such as genomics, transcriptomics, and proteomics to single cells to deeply analyze cell heterogeneity.
Spatial multi-omics: Combine molecular information with spatial location information to study the spatial structure and function of biological tissues.
Temporal multi-omics: Study the dynamic changes of biological processes by performing multi-omics analysis on biological systems at different time points.
2. Deeper biological problems
Analysis of complex traits: In-depth study of complex traits, such as cancer, neurodegenerative diseases, etc., to reveal the molecular mechanisms behind them.
Microbiome-host interaction: Explore the complex relationship between the microbiome and the host, and reveal the role of the microbiome in health and disease. For example, the application of multi-omics in covid-19.
Developmental biology: Study the development process of biological individuals from fertilized eggs to adults, and reveal the molecular mechanisms of developmental regulation.
3. Wider application areas
Precision medicine: Based on individual multi-omics data, accurate diagnosis and treatment of diseases can be achieved.
Environmental science: Monitor environmental pollution and assess the health of ecosystems.
Agriculture: Cultivate high-yield, pest-resistant crops and improve agricultural production efficiency.
4. Integration and analysis of multi-omics data
Artificial intelligence and machine learning: Use artificial intelligence and machine learning technologies to efficiently analyze massive multi-omics data and explore potential biological laws.
Data standardization: Establish a unified multi-omics data standard to facilitate data sharing and comparison between different research teams.
Cloud computing and big data technology: Use cloud computing and big data technology to store and analyze massive multi-omics data.
5. Combination of multi-omics with other technologies
Multi-omics and imaging: Combine multi-omics data with imaging data to achieve comprehensive analysis of biological tissues.
Multi-omics and clinical data: Combine multi-omics data with clinical data to establish a clinical prediction model.
Mahmood U, Li X, Fan Y, Chang W, Niu Y, Li J, Qu C and Lu K (2022) Multi-omics revolution to promote plant breeding efficiency. Front. Plant Sci. 13:1062952. doi: 10.3389/fpls.2022.1062952
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


