Methionine Metabolism: At the Crossroads of Methylation, Redox Balance, and Cellular Health
Among the twenty standard amino acids, methionine holds a unique and powerful position—not only as the initiating amino acid in protein synthesis, but also as a central player in methylation reactions, redox balance, and one-carbon metabolism. The metabolism of methionine is intricately connected to gene expression, antioxidant defense, and disease development, making it a key pathway of interest in both basic biology and clinical research.
What Is Methionine Metabolism?
Methionine metabolism refers to the series of enzymatic reactions through which methionine is activated, utilized, recycled, or degraded. As an essential amino acid, methionine must be obtained from dietary sources such as meat, fish, dairy, and legumes. Once inside the cell, methionine becomes more than just a building block—it is converted into S-adenosylmethionine (SAM), the universal methyl donor involved in the regulation of DNA, RNA, proteins, and lipids. Beyond methylation, methionine is also funneled into transsulfuration and polyamine synthesis pathways, affecting antioxidant defense and cell proliferation. The tightly regulated nature of this pathway ensures the balance between growth, detoxification, and epigenetic programming.
The Core Pathways of Methionine Metabolism
Methionine metabolism comprises several interconnected biochemical pathways that collectively influence methylation, redox homeostasis, and cellular biosynthesis. At its core, this network includes the methionine cycle, the transsulfuration pathway, and its close association with one-carbon and folate metabolism—each contributing uniquely to the cell's metabolic flexibility and physiological regulation.
The Methionine Cycle (SAM Cycle)
At the heart of methionine metabolism lies the methionine cycle, also known as the SAM (S-adenosylmethionine) cycle. This pathway begins when methionine, upon entering the cell, is activated by the enzyme methionine adenosyltransferase (MAT) to form S-adenosylmethionine (SAM). SAM is often referred to as the “universal methyl donor” because it transfers its methyl group to a vast array of substrates—including DNA, RNA, histones, phospholipids, and small molecules—through the action of various methyltransferases. These methylation reactions are central to epigenetic regulation, membrane fluidity, and biosynthetic processes.
After methyl group donation, SAM is converted into S-adenosylhomocysteine (SAH), which is subsequently hydrolyzed to yield homocysteine and adenosine. Homocysteine stands at a key metabolic junction. It can either be remethylated back to methionine, thus completing the cycle, or be directed into alternative pathways such as transsulfuration. The remethylation step is catalyzed by methionine synthase, which requires 5-methyltetrahydrofolate (5-MTHF) as a methyl donor and vitamin B12 as a cofactor, thereby linking the methionine cycle directly to folate metabolism. This cycle is critical not only for maintaining methylation capacity but also for regulating homocysteine levels and supporting metabolic flexibility under varying nutrient conditions.
_1745394056_WNo_1042d764.webp)
Methionine metabolism and related metabolic processes (Sanderson et al., 2019)
The Transsulfuration Pathway
When methionine intake is excessive or SAM levels are elevated, homocysteine is preferentially shunted away from remethylation and enters the transsulfuration pathway. This irreversible route serves a vital detoxification and antioxidant-supporting function. In the first step, homocysteine is condensed with serine by the enzyme cystathionine β-synthase (CBS) to form cystathionine. This intermediate is then cleaved by cystathionine γ-lyase (CSE) to produce cysteine, α-ketobutyrate, and ammonia.
Cysteine generated through this pathway is not only an important amino acid for protein synthesis but also serves as the limiting substrate for the production of glutathione (GSH), the major cellular antioxidant. Through this connection, the transsulfuration pathway becomes a critical node in redox homeostasis, helping cells adapt to oxidative stress by boosting their antioxidant capacity. Furthermore, defects in CBS or disruptions in this pathway can lead to elevated homocysteine levels (hyperhomocysteinemia), which are associated with increased risk for cardiovascular disease, neurodegeneration, and other chronic conditions. Thus, the transsulfuration pathway integrates methionine metabolism with the cell’s need to manage oxidative damage and maintain redox balance.
One-Carbon Metabolism and Folate Link
Methionine metabolism is deeply intertwined with one-carbon metabolism, a network of reactions responsible for transferring single-carbon units necessary for nucleotide synthesis, methylation, and amino acid interconversion. The connection between these two systems is primarily mediated through the remethylation of homocysteine to methionine, which requires 5-methyltetrahydrofolate (5-MTHF) from the folate cycle. This reaction is catalyzed by methionine synthase, with vitamin B12 acting as a crucial cofactor. As such, the availability of folate and B12 directly influences methionine regeneration and SAM availability.
Moreover, the folate cycle itself depends on the enzyme methylene tetrahydrofolate reductase (MTHFR) to produce 5-MTHF. Genetic variants in the MTHFR gene can impair this conversion, leading to reduced methylation potential and elevated homocysteine—both of which have been implicated in cardiovascular disease, neural tube defects, and neuropsychiatric disorders. One-carbon metabolism also supports the synthesis of purines and thymidylate, making it indispensable for DNA replication and repair. Therefore, the tight linkage between methionine and folate metabolism ensures that methylation processes, antioxidant defenses, and nucleotide biosynthesis are all finely tuned in response to cellular demands and nutrient status.
Functions and Significance of Methionine Metabolism
As a central hub connecting amino acid metabolism, epigenetic regulation, redox homeostasis, and biosynthesis, methionine metabolism plays diverse and essential roles in maintaining cellular function. Its significance goes far beyond protein synthesis, influencing how cells grow, differentiate, and respond to stress.
Methylation and Epigenetic Regulation
One of the most critical functions of methionine metabolism lies in the generation of S-adenosylmethionine (SAM), the primary methyl donor for a vast range of methylation reactions. These reactions are catalyzed by DNA, RNA, protein, and lipid methyltransferases, and are essential for epigenetic regulation, which governs gene expression without altering the DNA sequence. DNA methylation, for instance, plays a pivotal role in silencing repetitive elements, regulating gene accessibility, and maintaining chromatin structure. Similarly, histone methylation alters the architecture of chromatin and influences transcriptional activation or repression.
The delicate balance of SAM and its demethylated product, S-adenosylhomocysteine (SAH), is crucial in maintaining optimal methylation capacity. When SAH accumulates, it acts as a potent feedback inhibitor of methyltransferases, leading to global hypomethylation or abnormal hypermethylation patterns. Such disruptions in methylation homeostasis have been strongly linked to tumorigenesis, autoimmune diseases, and neurological disorders, where gene expression profiles are epigenetically misregulated. Thus, methionine metabolism serves as a metabolic-epigenetic interface that dynamically adjusts the cellular transcriptome in response to nutrient status and environmental cues.
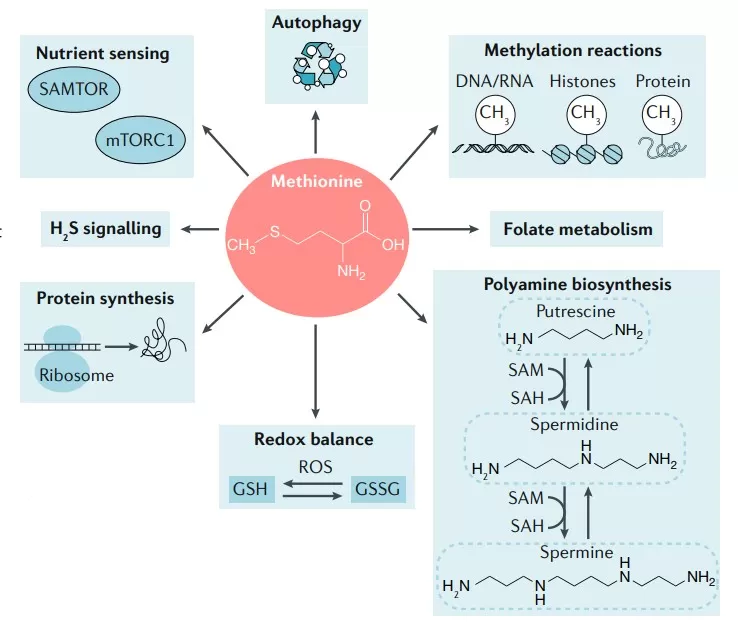
Role of methionine in biological processes (Sanderson et al., 2019)
Redox Homeostasis and Antioxidant Defense
In addition to its role in methylation, methionine metabolism contributes critically to cellular redox balance through the transsulfuration pathway. This pathway transforms homocysteine into cysteine, which is a precursor for the biosynthesis of glutathione (GSH)—the most abundant intracellular antioxidant. GSH neutralizes reactive oxygen species (ROS) and maintains redox-sensitive signaling molecules in their reduced states, thereby protecting cells from oxidative stress and maintaining cellular viability.
The demand for glutathione increases dramatically under conditions such as inflammation, ischemia, toxicity, and aging, all of which elevate ROS production. In these scenarios, the flux through the transsulfuration pathway must be upregulated to meet antioxidant demand. Therefore, methionine metabolism is not only a passive supplier of cysteine but also an active participant in adaptive stress responses. A deficiency in methionine or impaired CBS activity can lead to elevated homocysteine and reduced GSH synthesis, exacerbating oxidative damage and increasing the risk for cardiovascular and neurodegenerative diseases.
Cell Proliferation and Polyamine Biosynthesis
Methionine metabolism is also intimately involved in cell growth and proliferation, especially through its role in polyamine biosynthesis. Polyamines such as putrescine, spermidine, and spermine are small polycationic molecules that stabilize DNA, regulate gene expression, support membrane integrity, and modulate ion channels. Their synthesis begins with the decarboxylation of SAM, forming decarboxylated SAM (dcSAM), which then donates aminopropyl groups during polyamine formation.
This function becomes particularly critical in rapidly proliferating cells, including cancer cells, which exhibit a phenomenon known as methionine dependency or “methionine addiction.” These cells rely heavily on exogenous methionine for SAM production and are often more sensitive to methionine depletion than normal cells. This metabolic vulnerability has been explored as a therapeutic target in oncology, with methionine-restricted diets or inhibition of methionine-related enzymes demonstrating potential in slowing tumor growth, enhancing chemotherapy, and modulating the tumor microenvironment. Thus, methionine metabolism acts as a metabolic bottleneck for cell division and presents a strategic target for metabolic therapies.
Methionine Metabolism in Disease and Therapeutics
Alterations in methionine metabolism have been increasingly recognized as both drivers and biomarkers of disease. Its involvement spans cancer, cardiovascular pathology, and neurological disorders, often through mechanisms involving methylation imbalance, homocysteine accumulation, and redox dysfunction.
Cancer: Methionine Dependency and Epigenetic Reprogramming
Cancer cells frequently exhibit a phenomenon known as methionine dependency, where tumor cells are unable to proliferate in methionine-deficient environments, even when supplemented with homocysteine. This reflects their high reliance on exogenous methionine for sustained S-adenosylmethionine (SAM) synthesis, required to fuel DNA and histone methylation, polyamine synthesis, and other biosynthetic processes. Unlike normal cells, cancer cells often show hyperactive methylation demands due to epigenetic reprogramming and rapid gene expression shifts needed for proliferation.
Studies in glioblastoma, melanoma, and breast cancer have demonstrated that methionine restriction can suppress tumor growth, induce cell cycle arrest, and sensitize tumors to chemotherapy and immune checkpoint inhibitors. For example, a study reported that dietary methionine restriction enhanced the antitumor efficacy of immune therapy in mouse models by altering the tumor microenvironment and affecting histone methylation patterns. Furthermore, PET imaging using [11C]-methionine tracers has been clinically used to detect tumor metabolic activity, reflecting the diagnostic potential of targeting this pathway.
Cardiovascular Disease: Homocysteine as a Risk Factor
One of the most clinically relevant links between methionine metabolism and disease lies in hyperhomocysteinemia, a condition characterized by elevated plasma homocysteine levels due to impaired remethylation or transsulfuration. High homocysteine levels are a well-established independent risk factor for atherosclerosis, stroke, and coronary artery disease. The mechanisms are multifactorial and include endothelial dysfunction, oxidative stress, and increased thrombogenicity.
Population-based studies such as the Framingham Heart Study and the Homocysteine Studies Collaboration have shown that individuals with elevated homocysteine have up to a 2–3 fold increased risk of cardiovascular events. Mechanistically, homocysteine promotes LDL oxidation, reduces nitric oxide bioavailability, and enhances vascular inflammation. Genetic mutations in enzymes like MTHFR (e.g., C677T polymorphism) or nutritional deficiencies in folate, B6, or B12 can disrupt homocysteine clearance, exacerbating risk. While large-scale trials using B-vitamin supplementation have produced mixed results, targeted approaches in genetically predisposed individuals continue to be explored.
Neurological Disorders: Methylation and Neurodegeneration
Methionine metabolism also intersects with neurobiology, particularly through its role in methylation reactions essential for neuronal gene regulation, membrane composition, and neurotransmitter synthesis. SAM-dependent methylation is involved in myelin maintenance, synaptic function, and neurogenesis. Impairments in SAM availability or SAH accumulation can disturb DNA and histone methylation patterns, potentially contributing to cognitive decline, mood disorders, and neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease.
Elevated homocysteine has also been identified as a neurotoxin that may promote excitotoxicity, blood-brain barrier disruption, and increased oxidative damage in the central nervous system. Clinical studies have linked high homocysteine levels with poorer outcomes in dementia and depression. A meta-analysis concluded that lowering homocysteine through B-vitamin supplementation significantly slowed brain atrophy in individuals with mild cognitive impairment.
Furthermore, SAM supplementation has been investigated as a potential adjunct therapy for depression, with some randomized trials indicating comparable efficacy to conventional antidepressants in mild-to-moderate cases. This highlights the therapeutic potential of manipulating methionine and SAM metabolism to modulate brain chemistry and neuroplasticity.
Explore Methionine Metabolism with MetwareBio Metabolomics
To fully understand the complexity and regulatory significance of methionine metabolism in health and disease, precise and comprehensive metabolic profiling is essential. At MetwareBio, we offer comprehensive metabolomics solutions to support your methionine metabolism research. Our untargeted and widely targeted metabolomics platforms provide broad, unbiased coverage of metabolic changes, ideal for biomarker discovery and pathway mapping.
For focused investigations, our One-Carbon Metabolism Targeted Metabolomics Panel enables precise quantification of key metabolites such as methionine, SAM, SAH, homocysteine, cysteine, glutathione, and 5-MTHF. Whether you’re studying methylation, oxidative stress, or metabolic reprogramming, we deliver high-quality data and expert analytical support. Learn more at www.metwarebio.com
Reference
Sanderson, S. M., Gao, X., Dai, Z., & Locasale, J. W. (2019). Methionine metabolism in health and cancer: a nexus of diet and precision medicine. Nature reviews. Cancer, 19(11), 625–637. https://doi.org/10.1038/s41568-019-0187-8
Read more:
- Understanding Glycine: Its Metabolism and Vital Role in Human Well-Being
- Leucine: The Branched-Chain Amino Acid That Fuels Muscle Growth
- Unveiling Ornithine: Beyond the Urea Cycle, A Multifaceted Player in Health
- Malic Acid vs. Citric Acid: The Powerhouse Acids in Your Favorite Fruits
- Fumaric Acid Unveiled: From Nature's Palette to Therapeutic Potential
- Pyruvic Acid: A Key Player in Cellular Metabolism and Health
- Lactic Acid: Key Roles in Human Metabolism, Diseases, and Health Implications
- Cholic Acid: The Essential Bile Acid Impacting Digestion and Health
- Kynurenine: The Hidden Metabolite Linking Immunity, Mental Health, and Disease Prevention


