Choosing the Right Study Design for Metabolomics Biomarker Discovery
Metabolomics Biomarker Series Article Catalog:
- Unlocking Biomarkers: A Guide to Vital Health Indicators
- Metabolomics and Biomarkers: Unveiling the Secrets of Biological Signatures
All excellent scientific research results are based on a good "protocol design". It is, therefore, extremely important to develop a research protocol for metabolic marker screening. The first step in conducting a metabolomics clinical marker study is to recognize the types of studies that are commonly used in clinical research and to choose the one that best fits your research profile. In this section, the common types of clinical studies are summarized one by one. Clinical study designs can be categorized into two main types: those that involve passive observation of the target population are called observational studies, while those that involve active interventions are called experimental studies.
Observational studies can be subdivided into descriptive and analytical studies. Descriptive studies, i.e., descriptions of disease characteristics, prognosis, etc., include case reports and cross-sectional studies; analytical studies, i.e., studies of etiology and causation, or frequently influential factor analyses, include case-control studies and cohort studies.
Experimental studies are those in which the researcher consciously alters one or more factors that may be influential in the study, thereby verifying the effectiveness of this factor. They can be classified as randomized controlled trials and non-randomized controlled trials.
_1720422801_WNo_800d412.webp)
Among these clinical study designs, cross-sectional studies, case-control studies, cohort studies, and randomized controlled trials are the clinical study design scenarios that we apply the most with the technical tools of metabolomics, which are highlighted in the following sections.
1. Cross-sectional studies
Cross-sectional studies are designed to provide etiologic clues for further research by collecting and describing information on the distribution of disease or health conditions and related exposures in a population at a specific point in time and within a specific range. Only that point in time is investigated! No follow-up, no tracking!
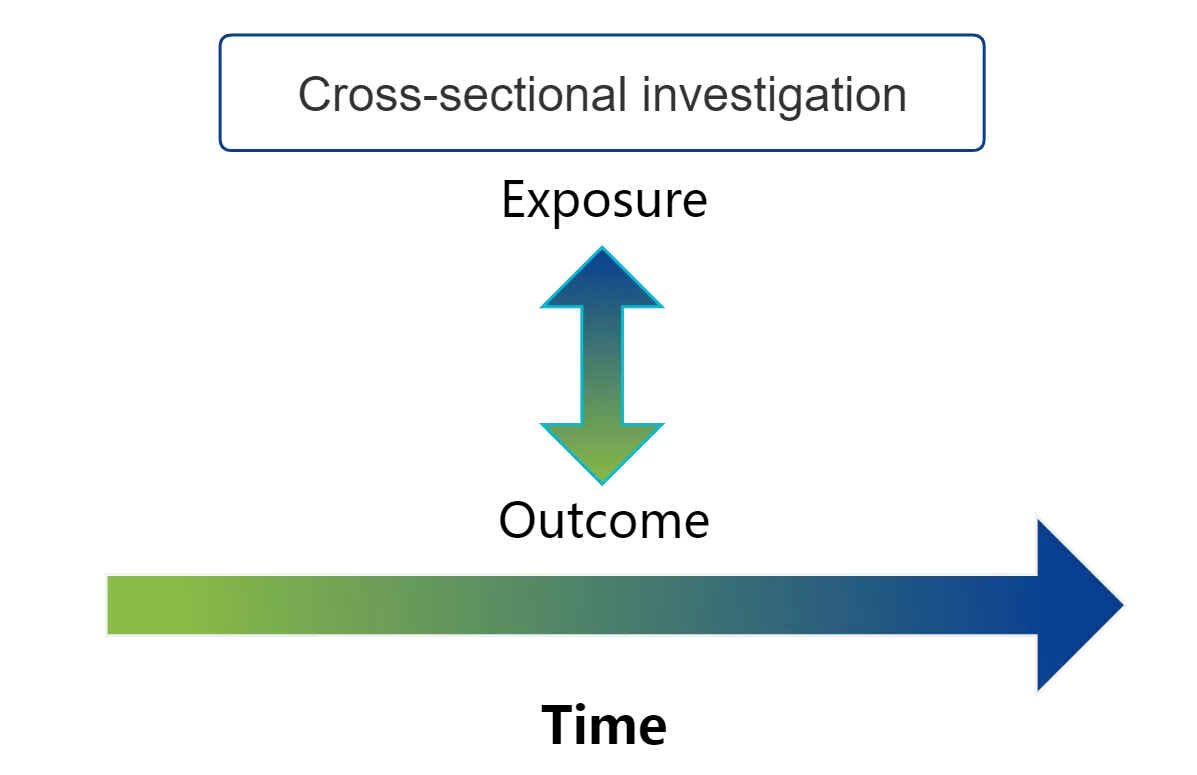 As the outcome and exposure are determined at the same time, the temporal relationship between the two is not clear, making it impossible to prove a causal relationship between them. For example, suppose a cross-sectional study found that men with gout had higher levels of glycine in their urine than men without gout. Did the elevated glycine cause the gout, or did the gout lead to metabolic disturbances that elevated the glycine levels? Such questions cannot be answered by cross-sectional studies, which is why cross-sectional studies are often used to establish hypotheses of causal relationships between clinical phenomena.
As the outcome and exposure are determined at the same time, the temporal relationship between the two is not clear, making it impossible to prove a causal relationship between them. For example, suppose a cross-sectional study found that men with gout had higher levels of glycine in their urine than men without gout. Did the elevated glycine cause the gout, or did the gout lead to metabolic disturbances that elevated the glycine levels? Such questions cannot be answered by cross-sectional studies, which is why cross-sectional studies are often used to establish hypotheses of causal relationships between clinical phenomena.
2. Case-control studies
 A case-control study is an observational study from 'effect' to 'cause' and is a typical retrospective study. This type of study first identifies case and control groups, followed by the retrospective collection of previous exposures. The presence and magnitude of the association between the investigated disease and factors are analyzed by comparing the exposure to the factor/risk factor of interest between the two populations prior to the study outcome.
A case-control study is an observational study from 'effect' to 'cause' and is a typical retrospective study. This type of study first identifies case and control groups, followed by the retrospective collection of previous exposures. The presence and magnitude of the association between the investigated disease and factors are analyzed by comparing the exposure to the factor/risk factor of interest between the two populations prior to the study outcome.
Case-control studies, which are easy to conduct and time-saving, are one of the most fundamental and important types of studies in epidemiology, serving as an indispensable tool for validating etiologic hypotheses.
3. Cohort studies
These are observational studies that move from 'cause' to 'effect', in which a specific population is divided into exposed and non-exposed groups (or multiple exposure level groups). These groups are followed up until the end of the study, with the differences in the incidence of the target outcome being compared between the groups, allowing for the determination of whether or not there is a causal association between the exposure factor and the outcome, and the magnitude of the association. In terms of the temporal dimension of data collection, cohort studies can be prospective, retrospective, or bidirectional.
3.1 Retrospective cohort studies
A retrospective cohort study is a study that takes time in the past as a starting point to collect baseline and exposure information. It divides the population into exposed and non-exposed groups based on their exposure to the study factors at that time and tracks the outcomes of morbidity or mortality up to the present time in order to study the relationship between the exposure and the disease. This design model is also known as a historical cohort study. Retrospective cohort studies presuppose that past records of exposure and morbidity are accurate and complete. Although the method of collecting exposure and outcome information is retrospective, it is still, by its nature, a cause-to-effect study.
3.2 Prospective cohort studies
A prospective cohort study is one in which observations are made from the present and followed to a future time for morbidity or mortality to determine the relationship between exposure and disease. This is what is usually referred to as a cohort study, and it is the basic form of cohort study.
3.3 Bidirectional cohort studies
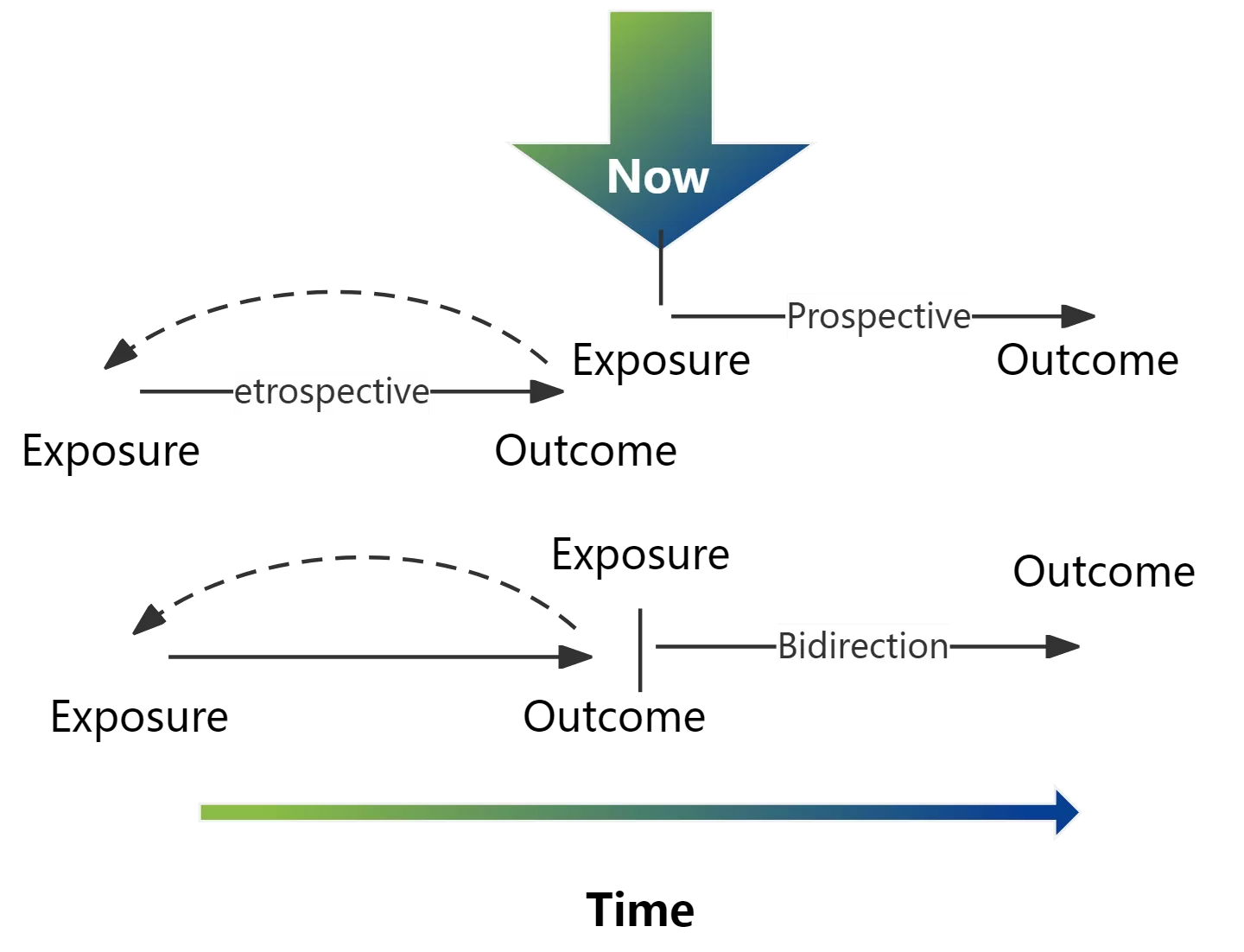 Bidirectional cohort studies are those that continue to follow-up beyond a retrospective cohort study to some future time. Also known as mixed cohort studies, they are a combination of prospective and retrospective cohort study methodologies, combining the strengths of both kinds and compensating for each other's shortcomings to a certain extent. In the course of conducting a retrospective cohort study, a bidirectional cohort study may be selected when sufficient observation time required to obtain the outcome cannot be realized from the time of exposure and a period of continued prospective observation is required.
Bidirectional cohort studies are those that continue to follow-up beyond a retrospective cohort study to some future time. Also known as mixed cohort studies, they are a combination of prospective and retrospective cohort study methodologies, combining the strengths of both kinds and compensating for each other's shortcomings to a certain extent. In the course of conducting a retrospective cohort study, a bidirectional cohort study may be selected when sufficient observation time required to obtain the outcome cannot be realized from the time of exposure and a period of continued prospective observation is required.
Either way, the logical direction of a cohort study is the same, i.e., from exposure to outcome.
Of the above three types, prospective cohort studies carry less bias and a higher level of evidence, but sometimes, a prospective study is not feasible in reality:
1) Prospective studies are generally not appropriate in cases where the incidence is low (low-risk events). For example, a certain adverse reaction to an antibiotic;
2) Prospective cohort studies should also not be selected where long-term follow-up (distant endpoint events) is required for definitive results. For example, the effect of growth hormone application in children on intelligence in adulthood or the effect of calcium supplements in childhood on the incidence of fractures in adulthood;
3) Prospective cohort studies are not advisable when the subject population has poor compliance. For example, observations of mild diseases, very severe diseases, or mobile populations.
Therefore, the choice is based on the actual conditions of the study.
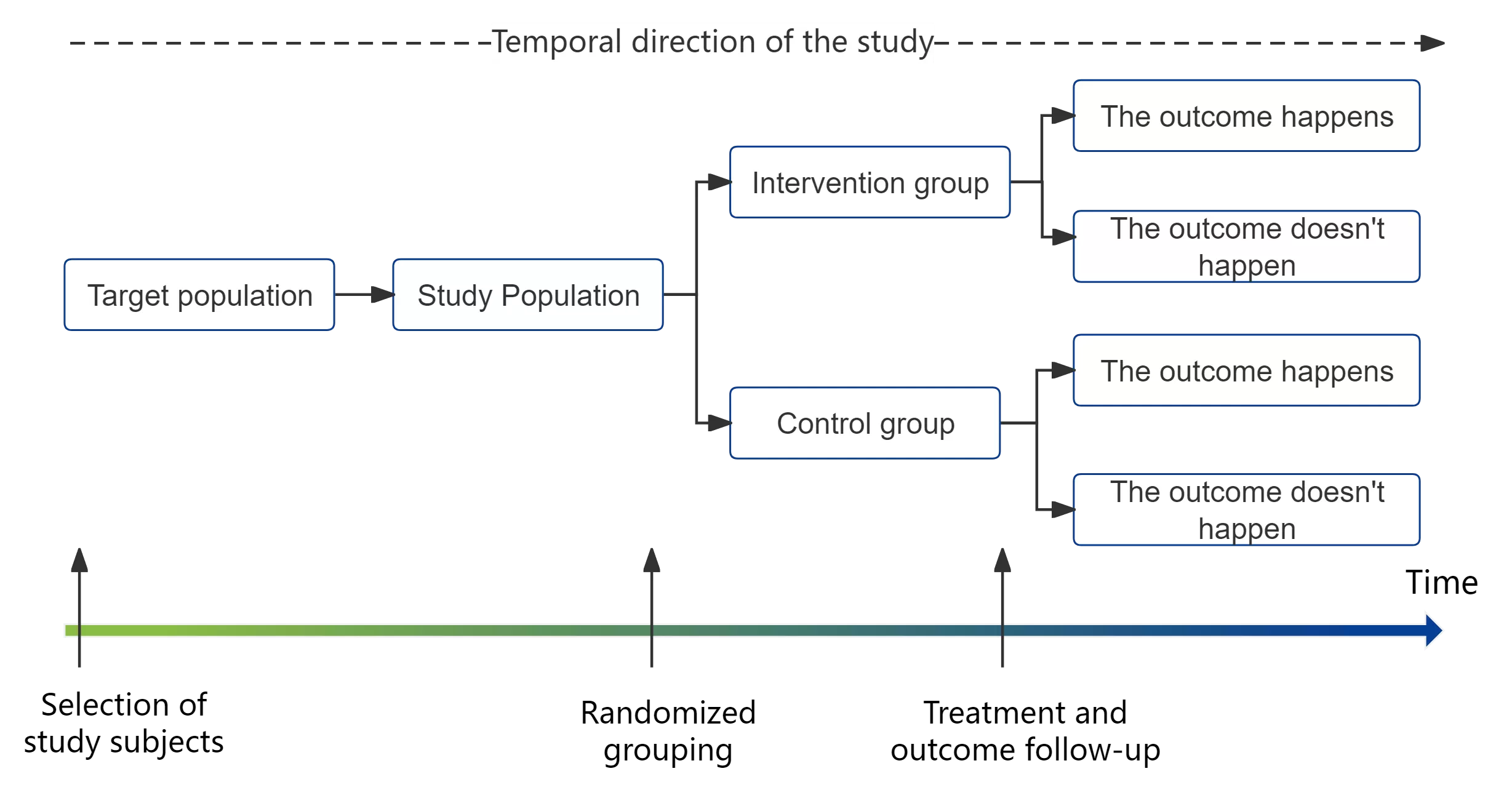
4. Randomized controlled trials
The experimental and control groups are grouped by using a truly randomized allocation method. It is a prospective study in which both groups are followed up for a specific period of time and then analyzed by pre-determined endpoint indicators.
Among experimental studies, randomized controlled trials (RCTs) have the highest level of reliability. RCTs are the only way to avoid selection bias and confounding factors in clinical studies and possess a high level of evidence. They are also a very well-known research type - e.g., RCTs are typically used in clinical trials concerning new drugs.
In clinical research, different experimental designs can be selected based on different clinical questions. For example, a cross-sectional study can be designed to find out the prevalence of disease (burden of disease) in a region; a case-control study and a cohort study can be designed to find out the causes or risk factors of disease; a cohort study can be designed to find out the prognosis and prognostic factors; and a randomized controlled study can be employed to find out the effect of a particular intervention.
|
Study type |
Study characteristics |
Study objectives |
Difficulty level |
|
|
Observational studies |
Case report |
Retrospective |
Problem exploration, hypothesis generation |
Easy |
|
Cross-sectional studies |
Status quo |
The current rate of something |
Determined by sample size and resources |
|
|
Case-control studies |
Retrospective (from effect to cause) |
Finding and validating disease risk factors |
Relatively easy |
|
|
Cohort studies |
Prospective cohort, retrospective cohort, bidirectional cohort (from cause to effect) |
Finding and validating risk factors, prognostic factors for diseases |
Relatively easy and with few restrictions on choices |
|
|
Experimental studies |
Randomized controlled studies |
Prospective (from cause to effect) |
Searching for effective interventions |
Difficult, costly, long lead time |
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


