Phosphatidylcholines: Unraveling the Essential Molecule
Phosphatidylcholines (PC) stand as quintessential molecules in the realm of cellular biology, forming the cornerstone of cell membranes and orchestrating a symphony of vital functions within the body. This introduction embarks on a journey to unravel the enigmatic essence of phosphatidylcholines, delving into their discovery, composition, biological significance, and various roles in health and disease.
- What is phosphatidylcholine?
- When and how was phosphatidylcholine discovered?
- How is phosphatidylcholine synthesized?
- What are the metabolism pathways of phophatidylcholine?
- Why is phophatidylcholine important to human?
- How can we absorb enough phophatidylcholine?
- Discover more about phophatidylcholine with MetwareBio!
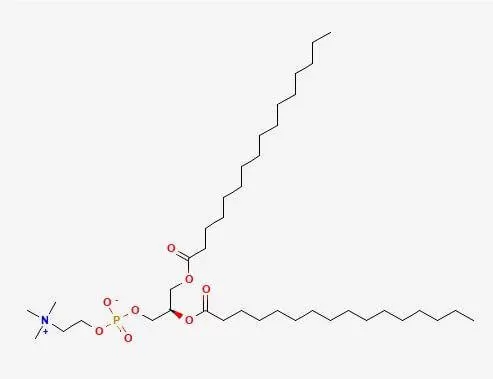
What is phosphatidylcholine?
Phosphatidylcholines (PC), a class of phospholipids crucial for cell membranes, possess a distinctive structure comprising a glycerol backbone, two fatty acid chains, a phosphate group, and a choline molecule, affording them both hydrophilic and hydrophobic properties pivotal for membrane formation. Integral to cellular functions like signaling, membrane fluidity, and lipid/protein transport, they safeguard membrane integrity and cellular contents. Acting as precursors for signaling molecules like diacylglycerol and inositol trisphosphate, PC regulates metabolic, proliferative, and apoptotic processes. Furthermore, PC's potential health benefits, evidenced in studies, have led to widespread use as dietary supplements, notably in lecithin form, sourced from soybeans and egg yolks, with implications for liver, brain, and cardiovascular health warranting further investigation.
When and how was phosphatidylcholine discovered?
 In the mid-19th century, the groundwork for understanding phosphatidylcholines began with the pioneering work of scientists such as Théodore Nicolas Gobley and Johann Friedrich Dieffenbach. Their investigations into the chemical composition of biological tissues laid the foundation for the discovery of phospholipids, of which phosphatidylcholines emerged as a prominent subclass.
In the mid-19th century, the groundwork for understanding phosphatidylcholines began with the pioneering work of scientists such as Théodore Nicolas Gobley and Johann Friedrich Dieffenbach. Their investigations into the chemical composition of biological tissues laid the foundation for the discovery of phospholipids, of which phosphatidylcholines emerged as a prominent subclass.
The true breakthrough came in the early 20th century when scientists such as Irving Langmuir and Herbert Gomberg elucidated the molecular structure of phosphatidylcholines. These molecules, characterized by a glycerol backbone, two hydrophobic fatty acid chains, a phosphate group, and a hydrophilic choline head, emerged as the fundamental building blocks of cell membranes, conferring them with remarkable stability and functionality.
How is phosphatidylcholine synthesized?
The biosynthesis of phosphatidylcholines (PC) is a complex process involving multiple steps and pathways within the cell. Here's an overview of the key steps and components involved in PC biosynthesis:
- Glycerophospholipid Synthesis Pathway: The de novo synthesis of PC typically begins with the glycerol-3-phosphate (G3P) pathway, which generates the backbone of phospholipids.
- Formation of CDP-Choline: In one major pathway, choline is activated to form CDP-choline (cytidine diphosphate choline) through the action of choline kinase (encoded by the CHKA gene) and cytidine triphosphate (CTP).
- Phosphatidylcholine Synthesis: CDP-choline reacts with diacylglycerol (DAG) in the presence of the enzyme phosphatidylcholine synthase (encoded by the Pcyt1 gene), resulting in the formation of phosphatidylcholine.
- Kennedy Pathway: Another significant pathway for PC synthesis is the Kennedy pathway, where phosphatidylcholine is synthesized from phosphatidylethanolamine (PE). In this pathway, phosphatidylethanolamine N-methyltransferase (PEMT) catalyzes the methylation of phosphatidylethanolamine to produce phosphatidylcholine, utilizing S-adenosylmethionine (SAM) as a methyl donor.
- Choline Uptake and Recycling: Choline, a precursor for PC synthesis, can be obtained from dietary sources or synthesized endogenously. Choline transporters, such as the high-affinity choline transporter (CHT1), facilitate choline uptake into cells. Additionally, choline can be recycled through the Kennedy pathway, contributing to PC synthesis.
- Regulation: PC biosynthesis is tightly regulated at multiple levels. Transcription factors such as sterol regulatory element-binding proteins (SREBPs) and liver X receptors (LXRs) regulate the expression of key enzymes involved in PC synthesis in response to cellular lipid and metabolic status.
- Integration with Other Pathways: PC biosynthesis is interconnected with other metabolic pathways, including glycolysis, fatty acid synthesis, and one-carbon metabolism. Metabolites such as SAM, which is involved in methylation reactions, play a crucial role in regulating PC synthesis.
._1723019923_WNo_939d558.webp)
Overall, the biosynthesis of phosphatidylcholines is a coordinated process involving multiple enzymes, substrates, and regulatory mechanisms, essential for maintaining membrane integrity and cellular function.
What are the metabolism pathways of phophatidylcholine?
Phosphatidylcholines (PC) metabolism encompasses various processes involved in the degradation, remodeling and integration of PC molecules within the cell. Here's a detailed overview of the key steps and components involved in PC metabolism:
1. Degradation of Phosphatidylcholines:
- Phospholipase A2 (PLA2) Pathway: PLA2 enzymes catalyze the hydrolysis of the sn-2 fatty acid ester bond in PC molecules, releasing a free fatty acid (usually arachidonic acid) and lysophosphatidylcholine (LPC). Arachidonic acid serves as a precursor for eicosanoid synthesis, while LPC can be further metabolized or recycled back into PC.
- Phospholipase C (PLC) Pathway: PLC enzymes cleave the phosphodiester bond of PC, generating DAG and a phosphorylated head group. DAG can be utilized in lipid biosynthesis pathways, while the phosphorylated head group may enter signaling pathways or be metabolized further.
- Phospholipase D (PLD) Pathway: PLD enzymes hydrolyze the phosphodiester bond of PC, producing phosphatidic acid (PA) and choline. PA serves as a precursor for the synthesis of other phospholipids or as a signaling molecule, while choline can be recycled for PC synthesis or utilized in other metabolic pathways.
2. Remodeling of Phosphatidylcholines:
- Acyltransferase Pathways: Lysophospholipid acyltransferases (LPLATs) catalyze the transfer of fatty acyl groups between PC molecules or between PC and other phospholipids, facilitating the remodeling of PC species with different fatty acid compositions.
- Phospholipase Pathways: Phospholipases, including PLA2 and phospholipase B (PLB), can also participate in PC remodeling by catalyzing the exchange or removal of fatty acids from PC molecules, leading to the generation of different PC species.
._1723019982_WNo_1022d588.webp)
3. Choline Metabolism:
- Betaine Pathway: Choline can be oxidized to betaine by choline dehydrogenase and betaine aldehyde dehydrogenase. Betaine serves as a methyl donor in the conversion of homocysteine to methionine, contributing to one-carbon metabolism and methylation reactions.
- Glycine Pathway: Betaine can be further metabolized to dimethylglycine and glycine, participating in various metabolic processes including protein synthesis and neurotransmitter metabolism.
4. Integration with Lipid Metabolism:
i. Metabolites generated during PC degradation and remodeling, such as DAG and LPC, can enter lipid biosynthesis pathways to replenish cellular lipid pools or serve as signaling molecules in various cellular processes.
ii. The metabolism of PC is interconnected with other lipid metabolic pathways, including glycerophospholipid metabolism, fatty acid metabolism, and lipid droplet dynamics, ensuring the coordination of lipid homeostasis within the cell.
These pathways collectively regulate the turnover and remodeling of phosphatidylcholines, allowing cells to dynamically adjust membrane composition and respond to changing metabolic demands and environmental cues.
Why is phophatidylcholine important to human?
Phosphatidylcholines (PC) play diverse roles in various diseases, influencing pathogenesis, progression, and potential therapeutic interventions. Here are some important findings regarding the functions of PC in diseases and their mechanisms:
1. Liver Diseases
Non-Alcoholic Fatty Liver Disease (NAFLD): In NAFLD, dysregulated PC metabolism contributes to hepatic lipid accumulation and inflammation. Reduced PC levels impair mitochondrial membrane integrity, compromising mitochondrial function and promoting lipid accumulation. Altered PC composition affects lipid droplet formation and hepatic lipid metabolism, exacerbating liver steatosis and inflammation. Furthermore, PC-derived lipids can act as signaling molecules, modulating inflammatory pathways and exacerbating liver injury.
2. Cardiovascular Diseases
Atherosclerosis: PC-rich lipoproteins, particularly high-density lipoprotein (HDL), play a crucial role in atherosclerosis prevention by promoting reverse cholesterol transport. PC molecules in HDL interact with ATP-binding cassette transporters, facilitating the efflux of cholesterol from macrophages in arterial walls to HDL particles. This process reduces foam cell formation and prevents the development of atherosclerotic plaques. Additionally, PC-rich lipoproteins possess antioxidant and anti-inflammatory properties, further contributing to their anti-atherogenic effects.
3. Neurological Disorders
Alzheimer's Disease (AD): In AD, alterations in PC metabolism contribute to synaptic dysfunction and neurodegeneration. Reduced PC levels and disrupted PC composition in neuronal membranes impair synaptic function, leading to synaptic loss and cognitive decline. Additionally, PC-derived lipids, such as lysophosphatidylcholine, can induce neuroinflammation and neurotoxicity, exacerbating neuronal damage and amyloid-beta accumulation. Modulating PC metabolism may restore synaptic integrity and mitigate neurodegenerative processes in AD.
4. Metabolic Disorders
Obesity and Insulin Resistance: Dysregulated PC metabolism contributes to obesity-related insulin resistance by impairing insulin signaling pathways. Altered PC composition in cell membranes disrupts insulin receptor localization and downstream signaling cascades, impairing glucose uptake and insulin sensitivity in adipocytes and skeletal muscle cells. Additionally, PC-derived lipids, such as diacylglycerol, can activate protein kinase C isoforms, further impairing insulin signaling and exacerbating insulin resistance.
5. Cancer
Hepatocellular Carcinoma (HCC): Aberrant PC metabolism promotes HCC progression by facilitating tumor cell proliferation and metastasis. Upregulated PC synthesis pathways, such as the Kennedy pathway and PEMT-mediated methylation, provide phospholipid precursors essential for membrane biogenesis and cell proliferation. Additionally, altered PC composition in tumor cell membranes affects membrane fluidity and signaling receptor localization, promoting oncogenic signaling and tumor cell survival. Targeting PC metabolism pathways may disrupt HCC growth and metastasis, offering therapeutic strategies for HCC treatment.
How can we absorb enough phophatidylcholine?
To ensure adequate absorption of phosphatidylcholine (PC), you can incorporate certain dietary sources rich in PC into your daily meals. Here are some tips to help you absorb enough phosphatidylcholine:
- Include Foods High in Phosphatidylcholine: Foods rich in PC include egg yolks, soybeans, sunflower seeds, peanuts, wheat germ, and organ meats like liver. Incorporate these foods into your diet regularly to increase your intake of PC.
- Consume Lecithin Supplements: Lecithin supplements are a concentrated source of phosphatidylcholine. They are available in various forms, such as capsules, granules, or liquid. Taking lecithin supplements with meals can enhance the absorption of PC.
- Choose Whole Foods: Opt for whole, minimally processed foods over highly processed options. Whole foods contain higher amounts of naturally occurring phospholipids, including phosphatidylcholine, compared to processed foods.
- Cook Foods Gently: Cooking methods such as steaming, boiling, or lightly sautéing foods help preserve the phospholipid content, including phosphatidylcholine, in foods like vegetables and meats.
- Incorporate Healthy Fats: Phosphatidylcholine is a type of phospholipid found in dietary fats. Including sources of healthy fats such as olive oil, avocados, fatty fish, and nuts in your diet can support the absorption of phosphatidylcholine and other phospholipids.
- Consider Digestive Enzymes: Some individuals may have digestive issues that affect the absorption of phospholipids. Digestive enzyme supplements containing lipase can aid in the breakdown and absorption of fats, including phospholipids like phosphatidylcholine.
- Balance Your Diet: Aim for a well-balanced diet that includes a variety of nutrients, including phospholipids like phosphatidylcholine. Consuming a diverse range of foods ensures that you receive an adequate intake of essential nutrients, including PC.
Discover more about phophatidylcholine with MetwareBio!
As a leading metabolomics service provider, MetwareBio offers comprehensive lipidomics services tailored to researchers' needs. Our cutting-edge technologies and expertise enable precise analysis and characterization of phosphatidylcholine (PC) and its metabolites, providing valuable insights into lipid metabolism and its implications in health and disease. By partnering with MetwareBio, researchers gain access to state-of-the-art analytical techniques, including liquid chromatography-mass spectrometry (LC-MS), and bioinformatics analysis tools, allowing for in-depth exploration of PC profiles in biological samples. Whether investigating the role of PC in liver diseases, neurological disorders, or metabolic conditions, our dedicated team is committed to delivering high-quality data and actionable insights to advance your research. Join us in unraveling the mysteries of phosphatidylcholine and unlocking new opportunities for scientific discovery with MetwareBio!
References:
1. Dushianthan A, Cusack R, Grocott MPW, Postle AD. Abnormal liver phosphatidylcholine synthesis revealed in patients with acute respiratory distress syndrome. J Lipid Res. 2018;59(6):1034-1045. doi:10.1194/jlr.P085050
2. Whiley L, Sen A, Heaton J, et al. Evidence of altered phosphatidylcholine metabolism in Alzheimer's disease. Neurobiol Aging. 2014;35(2):271-278. doi:10.1016/j.neurobiolaging.2013.08.001


