Mastering Chromatography: Everything You Need to Know
In today’s world, where precision and accuracy are essential, chromatography stands as a cornerstone technique in scientific research and industry. Whether it's in pharmaceuticals, environmental science, or food quality control, chromatography is crucial for separating and analyzing complex mixtures. But what exactly is chromatography, and how does it work?
This blog delves into the fascinating world of chromatography, offering an in-depth exploration of its principles, history, types, and practical applications. Whether you're a student, researcher, or industry professional, understanding chromatography can open doors to a wealth of knowledge across various scientific disciplines. Join us as we take a closer look at how this powerful technique helps unlock the secrets hidden within complex samples and why it remains an indispensable tool for modern science.
- What is Chromatography?
- Historical Background of Chromatography
- Principles of Chromatography
- Types of Chromatography
- Components and Instrumentation of Chromatography
- Applications of Chromatography
1. What is Chromatography?
Chromatography is a versatile analytical technique used to separate, identify, and quantify the components of a mixture. At its core, the process relies on the differential movement of individual components between two phases: the stationary phase (a solid or a liquid immobilized on a solid surface) and the mobile phase (a liquid or gas that flows through or over the stationary phase). As the mixture interacts with these two phases, its components travel at different speeds, leading to their separation.
The term "chromatography" comes from the Greek words chroma (color) and graphein (to write). It was coined by the Russian botanist Mikhail Tsvet in the early 20th century, who used the technique to separate pigments in plant extracts, creating a vivid visual display of separated colors.
Chromatography plays a critical role in drug development by ensuring medication purity and safety, monitoring environmental pollutants, and verifying the quality of food products. Its versatility and accuracy make it an indispensable tool across chemistry, biology, and various industries.
2. Historical Background of Chromatography
The origins of chromatography date back to the early 20th century when Russian botanist Mikhail Tsvet first introduced the technique in 1906. Tsvet was studying plant pigments, particularly chlorophyll, and discovered that by passing a plant extract through a glass column packed with calcium carbonate, the pigments separated into distinct colored bands. He called this method "chromatography," meaning "color writing," due to the visual separation of pigments. This groundbreaking work laid the foundation for modern chromatographic methods.
For decades, chromatography remained a niche technique until the mid-20th century, when its potential was fully realized with the development of gas chromatography (GC) in the 1940s and high-performance liquid chromatography (HPLC) in the 1960s. These advancements significantly expanded its applications, making it indispensable in chemical, pharmaceutical, and environmental research.
Modern chromatography has since evolved into a sophisticated analytical science. With innovations such as automated instrumentation and the integration of mass spectrometry, it is now capable of detecting and analyzing substances at trace levels, pushing the boundaries of scientific discovery and industrial application.
From its humble beginnings as a simple method for separating plant pigments to its current role as a cornerstone of analytical chemistry, chromatography has had a profound impact on science and industry.
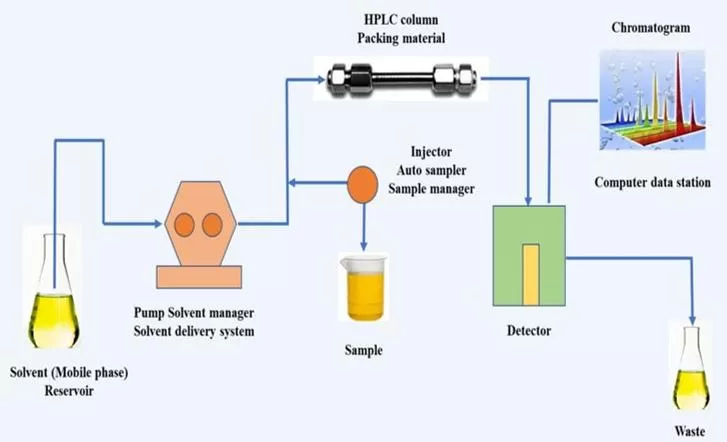
Schematic Representation of High-Performance Liquid Chromatography
3. Principles of Chromatography
Chromatography is based on the fundamental principle of separation through differential interactions. A mixture’s components are distributed between two phases: a stationary phase and a mobile phase. The way these components interact with each phase determines how they are separated.
The Two Phases of Chromatography
1) Stationary Phase: This is a fixed phase, often a solid or a liquid coated on a solid support. It provides the surface for interactions with the components of the mixture.
2) Mobile Phase: This is a fluid, either a liquid or a gas, that flows through or over the stationary phase, carrying the mixture’s components.
The differential affinity of each component for the stationary and mobile phases causes them to move at different speeds, leading to their separation.
Mechanisms of Separation
The separation process depends on how each component interacts with the stationary and mobile phases. Common mechanisms include:
1) Adsorption: Components adhere to the surface of the stationary phase.
2) Partition: Components distribute themselves between the stationary and mobile phases based on their solubility.
3) Size Exclusion: Components are separated by size as they pass through a stationary phase with pores of specific dimensions.
4) Ion Exchange: Components are separated based on their charge through interactions with charged sites on the stationary phase.
Each mechanism is used for specific types of analyses, depending on the chemical and physical properties of the mixture.
Key Factors Influencing Separation
Several factors influence how effectively a mixture’s components are separated:
1) Affinity Differences: The strength of interaction with the stationary phase versus solubility in the mobile phase.
2) Flow Rate: The speed of the mobile phase affects resolution and separation time.
3) Temperature: In methods like gas chromatography, temperature influences component volatility and separation efficiency.
Optimizing these factors ensures accurate and efficient separation in analytical or preparative applications.
Chromatography is, at its heart, a methodical and precise process that relies on the balance of physical and chemical principles. By understanding these principles, scientists can tailor the technique to suit a wide range of analytical needs, from isolating a single compound to profiling complex mixtures.
4. Types of Chromatography
Chromatography encompasses a wide range of techniques, each tailored to specific types of mixtures and analytical needs. These techniques are classified based on the physical state of the mobile phase, the separation mechanism, or the scale of operation.
Classification by Physical State of the Mobile Phase
1) Gas Chromatography (GC): Gas chromatography uses a gaseous mobile phase to separate volatile compounds. The stationary phase is typically a liquid film coated on a solid support or the walls of a capillary column. It is widely used in analyzing environmental pollutants, essential oils, and petroleum products. It is particularly effective for volatile and thermally stable compounds.
2) Liquid Chromatography (LC): Liquid chromatography employs a liquid mobile phase to separate components based on their solubility and interaction with the stationary phase. It is used in pharmaceutical analysis, food quality testing, and biotechnology. High-performance liquid chromatography (HPLC) is the most advanced form, enabling high-resolution separations.
3) Supercritical Fluid Chromatography (SFC): SFC uses a supercritical fluid (e.g., carbon dioxide above its critical point) as the mobile phase, combining the advantages of GC and LC. It is common in chiral separations, especially in the pharmaceutical industry.
Classification by Separation Mechanism
1) Adsorption Chromatography: Components are separated based on their affinity for the surface of a solid stationary phase. This technique is used in thin-layer chromatography (TLC) and is often employed for qualitative analysis of organic compounds and reaction monitoring.
2) Partition Chromatography: Separation occurs based on the differential distribution of components between a liquid stationary phase and a liquid mobile phase, depending on their solubility. Paper chromatography is a classic example and is often used in biochemical applications, such as amino acid or protein separations.
3) Ion-Exchange Chromatography: Components are separated based on ionic interactions between the charged analytes and the charged sites on the stationary phase. It is commonly used for water purification, protein purification, and separating charged biomolecules.
4) Size-Exclusion Chromatography (SEC): Separation is based on the size of the molecules as they pass through a porous stationary phase. This technique is useful for determining molecular weight distributions in polymers and purifying macromolecules such as proteins.
5) Affinity Chromatography: Components are separated based on specific interactions with a stationary phase that has been functionalized with a ligand or receptor. It is widely used in biotechnology to isolate enzymes, antibodies, and other biomolecules.
Classification by Scale of Operation
- Analytical Chromatography: This scale is primarily focused on the identification and quantification of components in a mixture. It is used in routine laboratory analysis across various fields such as forensics, pharmaceuticals, and environmental testing.
- Preparative Chromatography: This scale is designed for the isolation and purification of larger quantities of a specific compound. It is commonly used in industrial-scale processes like drug manufacturing and natural product extraction.
5. Components and Instrumentation of Chromatography
Chromatography systems consist of several key components that work together to facilitate the separation and detection of components in a mixture. These components can vary depending on the specific type of chromatography being used, but the basic elements remain largely the same.
The Column
The column is a critical component in most chromatography techniques. It contains the stationary phase, which interacts with the sample components as they move through the column.
1) Capillary Columns: Used in gas chromatography (GC) and sometimes in liquid chromatography (LC), these columns have an inner coating of the stationary phase.
2) Packed Columns: Typically found in preparative chromatography, these columns are filled with solid particles that support the stationary phase.
The column’s length, diameter, and material are chosen based on the specific application and the type of chromatography.
The Mobile Phase Delivery System (Pump)
The mobile phase (liquid or gas) must be delivered at a constant and controlled flow rate. The pump performs this task in liquid chromatography (LC), ensuring a steady flow of solvent.
In Gas Chromatography (GC), a pressurized gas, typically helium or nitrogen, is used to push the sample through the column. In High-Performance Liquid Chromatography (HPLC), advanced pumps are designed to operate at high pressures to push liquid mobile phases through tightly packed columns for high-resolution separations.
The Injector
The injector introduces the sample mixture into the chromatography system. In GC, a small sample is injected into a heated port, where it vaporizes and enters the column. In LC, the sample is typically dissolved in a solvent and injected through a syringe or an automatic injector system into the liquid mobile phase. The injector must be precise to ensure that the sample is introduced in a controlled manner, without affecting the separation.
The Detector
The detector is used to identify and quantify the separated components as they elute (exit) from the column. There are various types of detectors, each suited to different types of chromatography:
1) UV/Visible Detectors: Common in liquid chromatography (LC), these detectors measure the absorption of ultraviolet or visible light by the sample as it passes through a flow cell.
Fluorescence Detectors: Sensitive to compounds that fluoresce under UV light, these detectors are often used in HPLC for detecting trace levels of substances.
2) Mass Spectrometry (MS): When coupled with chromatography (e.g., GC-MS, LC-MS), mass spectrometers provide detailed structural information and high sensitivity for identifying components at low concentrations.
3) Thermal Conductivity Detector (TCD): Common in GC, this detector measures the change in the thermal conductivity of the mobile phase as components exit the column.
The choice of detector depends on the chemical properties of the analytes and the sensitivity required.
Data Collection and Analysis System
Modern chromatography systems are equipped with sophisticated data collection and analysis systems. These systems record and analyze the detector’s output, generating chromatograms that plot the detector response versus time. Chromatographic software is used to process and analyze the data, identifying peaks corresponding to individual components, calculating their concentrations, and providing further analytical results. The software also integrates the peaks to calculate the area under the curve, which corresponds to the quantity of a given analyte in the sample.
6. Applications of Chromatography
Chromatography is an indispensable tool across a wide range of industries and scientific fields due to its versatility and precision. It is used for separating and analyzing complex mixtures in various applications, from pharmaceuticals to environmental monitoring.
1. Pharmaceutical Industry
Chromatography is essential in the pharmaceutical industry for both the development and quality control of drugs.
Drug Purity: Techniques like HPLC and GC are used to ensure the purity of drugs by separating active ingredients from impurities or degradation products.
Bioanalytical Applications: Chromatography is used to measure blood levels of pharmaceutical compounds, ensuring proper dosing.
Chiral Separations: For drugs that exist as enantiomers, chromatography helps in separating and analyzing the active form, which is crucial for drug efficacy and safety.
2. Environmental Monitoring
Chromatography plays a vital role in environmental science by detecting pollutants and ensuring compliance with regulatory standards.
Water and Soil Testing: GC and HPLC are used to detect contaminants such as pesticides, heavy metals, and hydrocarbons in water and soil samples.
Air Quality Monitoring: Chromatographic techniques are used to analyze air samples for pollutants, including volatile organic compounds (VOCs) and greenhouse gases.
Environmental Toxicology: Chromatography helps identify the presence of toxins in ecosystems, including industrial waste and agricultural runoff.
3. Food and Beverage Industry
Ensuring the safety, quality, and authenticity of food products is a major application area for chromatography.
Food Safety: Chromatography is used to detect contaminants such as pesticides, herbicides, and food additives in food products.
Quality Control: Techniques like HPLC are used to monitor the quality and consistency of ingredients, such as the concentration of vitamins or preservatives in food.
Flavor and Aroma Analysis: GC is widely used for the analysis of volatile compounds in food and beverages, ensuring that flavors and aromas are consistent and safe for consumption.
4. Forensic Science
In forensic science, chromatography is employed to analyze biological and chemical evidence from crime scenes.
Toxicology: Chromatography techniques, such as GC-MS, are used to identify drugs, poisons, and other toxic substances in blood, urine, and tissue samples.
Controlled Substance Analysis: It helps in identifying illicit drugs and their metabolites, providing evidence in criminal investigations.
Trace Evidence: Chromatography can also be used to identify trace chemicals or substances found at crime scenes, such as explosives or accelerants.
5. Biotech and Biomedical Research
Chromatography is a key tool in biotechnology and biomedical research, particularly in the isolation and analysis of biomolecules.
Protein Purification: Affinity chromatography is commonly used to isolate specific proteins or antibodies for therapeutic or research purposes.
Metabolomics and Proteomics: Chromatography helps in analyzing metabolites and proteins, aiding in disease research and biomarker discovery.
Vaccine Development: During the development of vaccines, chromatography helps purify antigens and ensure their safety and effectiveness.
6. Petrochemical Industry
Chromatography is used in the petrochemical industry to analyze hydrocarbons and monitor the composition of fuels and oils.
Gasoline and Diesel Quality Control: GC is used to analyze the composition of fuels, checking for contaminants and ensuring quality.
Crude Oil Analysis: Chromatography helps in analyzing crude oil and refining processes, providing insights into the chemical makeup and potential yield of different fractions.
Reference
Bhati C, Minocha N, Purohit D, Kumar S, Makhija M, Saini S, Kaushik D, Pandey P. High Performance Liquid Chromatography: Recent Patents and Advancement. Biomed Pharmacol J 2022;15(2).
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


