Malic Acid vs. Citric Acid: The Powerhouse Acids in Your Favorite Fruits
Have you ever wondered what makes apples so tangy or why citrus fruits like lemons and oranges have that signature zest? The answer lies in two powerhouse acids—malic acid and citric acid—which play a crucial role not only in the taste of these fruits but also in our overall health. These acids are far more than just flavor enhancers; they are key players in our metabolism, energy production, and disease prevention.
In this blog, we’ll dive into the science behind malic and citric acids, starting with their discovery and structure. We’ll explore where they come from and how they’re synthesized in our body. You’ll also discover their essential roles in energy production, muscle function, and detoxification, and how these acids contribute to the prevention and management of diseases like chronic fatigue, cardiovascular conditions, and kidney stones. Finally, we’ll touch on the methods used to analyze malic and citric acids in scientific research. Whether you’re curious about how these acids benefit your health or how they’re used in everyday fruits, this guide will provide a thorough understanding of their significance in both nature and our bodies.
- What is Malic Acid? Discovery, Structure and Sources
- What is Citric Acid? Discovery, Structure and Sources
- Synthesis and Metabolic Pathways of Malic Acid and Citric Acid
- Biological Functions and Roles of Malic Acid and Citric Acid
- Malic and Citric Acids in Disease Prevention and Management
What is Malic Acid? Discovery, Structure and Sources
Malic acid, often referred to in its ionic form as malate, is a naturally occurring organic compound that plays a vital role in metabolism, particularly in the process of energy production. Known for its tart taste, malate is found in a variety of fruits, with apples being one of its richest sources, giving them their characteristic sour flavor.
Discovery of Malic Acid (malate)
Malic acid was first isolated in 1785 by the Swedish chemist Carl Wilhelm Scheele from apple juice. The name "malic" comes from the Latin word malum, meaning "apple," reflecting its presence in this fruit. Over time, malic acid (or malate in its deprotonated form) was recognized as a crucial component in plant and animal metabolism, especially as scientists understood its role in the citric acid cycle (also known as the Krebs cycle), which is essential for producing energy in cells.
Chemical Structure of Malic Acid (malate)
Malic acid, or malate when in its ionized form, is a dicarboxylic acid, meaning it contains two carboxyl groups (COOH). Its molecular formula is C₄H₆O₅, and its structure consists of a four-carbon backbone with two carboxyl groups and a hydroxyl group attached to one of the carbons. When malic acid loses a proton, it becomes malate, the form that is typically found in the citric acid cycle. This structure allows malate to participate in key biochemical pathways, such as the citric acid cycle, where it is involved in the conversion of food into usable energy. The structure of malate also gives it its acidic properties, making it an important molecule for the sourness in various fruits and beverages, as well as for its metabolic functions.
_1737600994_WNo_624d350.webp)
Figure 1. The chemical structure of malic acid (image adapted from PubChem)
Sources of Malic Acid (malate)
Malic acid is found in many fruits and vegetables, with the highest concentrations in apples. It is also abundant in cherries, grapes, pears, apricots, and plums, which contribute to the tartness of these fruits. Beyond fruits, malic acid is also present in certain vegetables, such as tomatoes and potatoes, though in lower amounts.
In addition to natural sources, malic acid is often used as a food additive and flavor enhancer, particularly in sour candies, soft drinks, and other processed foods. It's also found in a variety of health supplements, where it's touted for its potential benefits in energy production and muscle recovery.
What is Citric Acid? Discovery, Structure and Sources
Citric acid, also known as citrate when ionized, is one of the most important organic acids found in living organisms. It is a key player in the production of energy within our cells and is most commonly associated with citrus fruits like lemons and oranges, where it contributes to their tangy, refreshing flavor. Beyond its role in flavor, citric acid (or citrate) plays a central role in metabolism, especially in the citric acid cycle (Krebs cycle), which is essential for cellular energy production.
Discovery of Citric Acid (Citrate)
Citric acid was first discovered in 1784 by the Swedish chemist Carl Wilhelm Scheele from lemon juice, giving rise to its association with citrus fruits. The name "citric" is derived from the Latin word citrus, meaning "lemon." Later, citric acid was identified as a crucial component in the process of cellular respiration. The transformation of citric acid into citrate (its ionized form) plays a pivotal role in the citric acid cycle, where it facilitates the conversion of carbohydrates and fats into energy within the mitochondria of cells.
Chemical Structure of Citric Acid (Citrate)
Citric acid, when in its natural acidic form, is a tricarboxylic acid, meaning it contains three carboxyl groups (COOH). Its molecular formula is C₆H₈O₇, and its structure consists of a six-carbon backbone with three carboxyl groups, one hydroxyl group, and a central carbon atom. This structure allows citric acid to be a highly effective molecule for energy production. When citric acid loses a proton (H⁺), it becomes citrate, the form that is typically found in metabolic pathways like the citric acid cycle. The conversion of citric acid into citrate is a crucial step in many biochemical reactions, as it allows citric acid to function as an intermediate molecule in energy metabolism.
_1737601046_WNo_626d510.webp)
Figure 2. The chemical structure of citric acid (image adapted from PubChem)
Sources of Citric Acid (Citrate)
Citric acid, or citrate, is most abundant in citrus fruits like lemons, oranges, grapefruits, and limes, which is why these fruits have a distinctly sour taste. It is also found in lesser amounts in other fruits such as pineapples, strawberries, and raspberries, as well as in some vegetables, including tomatoes and peppers.
Beyond its natural occurrence, citric acid is widely used in the food and beverage industry as a preservative and flavor enhancer. It's commonly added to soft drinks, candies, jams, and processed foods to provide a tart, refreshing taste. Citric acid is also a key ingredient in cleaning products, pharmaceuticals, and cosmetics, thanks to its ability to act as a natural preservative and pH regulator.
Table 1. Comparison of Key Properties of Malic Acid and Citric Acid
|
Property |
Malic Acid |
Citric Acid |
|
Chemical Formula |
C₄H₆O₅ |
C₆H₈O₇ |
|
Molecular Weight |
134.09 g/mol |
192.13 g/mol |
|
Taste/Flavor |
Sour, tart, smooth, mild tartness |
Sharp, tangy, intense sourness |
|
pH |
Typically 3.0 - 3.5 |
Typically 2.2 - 2.4 |
|
Structure |
Dicarboxylic acid |
Tricarboxylic acid |
|
Sources |
apples, cherries, peaches, and other fruits and vegetables |
citrus fruits, and some berries and vegetables |
Synthesis and Metabolic Pathways of Malic Acid and Citric Acid
Malic acid and citric acid are central to the energy production process in living organisms, particularly through their involvement in the tricarboxylic acid cycle (TCA cycle). Both acids participate in key biochemical pathways that enable cells to convert carbohydrates, fats, and proteins into ATP, the primary energy currency of the cell.
The TCA Cycle: A Shared Pathway for Malic and Citric Acids
The TCA cycle, which occurs in the mitochondria, is often referred to as the citric acid cycle due to the involvement of citric acid in the cycle's initial steps. The TCA cycle begins when acetyl-CoA, derived from carbohydrates, fats, or proteins, combines with oxaloacetate to form citric acid (or citrate). Citrate then undergoes a series of transformations: first, it is isomerized to form isocitrate, followed by a decarboxylation step where isocitrate is converted into α-ketoglutarate, releasing one molecule of CO₂ and producing NADH. Next, α-ketoglutarate undergoes another decarboxylation, forming succinyl-CoA while producing more NADH and releasing another CO₂. Succinyl-CoA is then converted into succinate, generating GTP (or ATP) in the process. Succinate is oxidized to fumarate, producing FADH₂. Finally, fumarate is hydrated to form malate, which is then converted back into oxaloacetate, regenerating the cycle and producing another molecule of NADH. This continuous process generates high-energy molecules like NADH, FADH₂, and GTP, which fuel the cell’s energy needs.
_1737601133_WNo_850d902.webp)
Figure 3. Illustration of the citric acid cycle, or Krebs cycle (Lehninger et al., 2013)
Alternative Pathways for Malic and Citric Acid Synthesis
In addition to their roles in the TCA cycle, malic acid and citric acid are synthesized through other important metabolic pathways. Malic acid can be synthesized in plants during C4 photosynthesis, a process that allows certain plants (like corn and sugarcane) to efficiently fix carbon dioxide. In this pathway, phosphoenolpyruvate (PEP) reacts with carbon dioxide, forming oxaloacetate, which is then converted to malate by malate dehydrogenase. This malate is stored in vacuoles and later decarboxylated to release CO₂ during the Calvin cycle. Additionally, malic acid is produced in the malate-aspartate shuttle, where it is formed from oxaloacetate in the cytoplasm, facilitating the transfer of electrons across the mitochondrial membrane, crucial for energy production during aerobic respiration.
_1737601200_WNo_885d480.webp)
Figure 4. Schematic of the malate aspartate shuttle (Koch et al., 2024)
Similarly, citric acid can be synthesized through alternative pathways outside the TCA cycle, particularly in microorganisms. In citric acid fermentation, Aspergillus niger, a mold, utilizes acetate or acetyl-CoA to produce citric acid. This process bypasses the TCA cycle and is used for industrial-scale production of citric acid. Moreover, in certain plants and bacteria, citric acid can be synthesized from glutamate, an amino acid. In this pathway, glutamate is converted to α-ketoglutarate, which enters the TCA cycle, eventually leading to the production of citric acid. These alternative pathways ensure that malic acid and citric acid are available for a variety of cellular processes, such as energy production, biosynthesis, and carbon fixation.
Biological Functions and Roles of Malic Acid and Citric Acid
Malic acid and citric acid are integral to various physiological processes within the body. Their roles extend beyond being intermediates in the TCA cycle to include vital functions in energy production, oxidative stress regulation, pH balance, and biosynthesis. These acids not only contribute to cellular metabolism but also help maintain overall health by supporting critical biochemical pathways.
Energy Production and Cellular Metabolism
Both malic acid and citric acid are central to the process of cellular energy production, primarily through their involvement in the TCA cycle (also known as the Krebs cycle). In the cycle, citric acid is the initial product formed when acetyl-CoA combines with oxaloacetate, and it undergoes several transformations to release energy in the form of ATP, NADH, and FADH₂. These energy carriers are critical for fueling cellular processes that require energy, such as biosynthesis, cell division, and maintenance of cellular structures. Malic acid, formed during the cycle, also contributes to this process by being converted back into oxaloacetate, which then continues the cycle. As intermediates in this essential metabolic pathway, both acids are directly linked to maintaining energy homeostasis within cells.
Antioxidant Defense and Oxidative Stress Regulation
Malic acid and citric acid also play important roles in protecting cells from oxidative stress—a condition characterized by the excess production of reactive oxygen species (ROS) that can damage cellular components. Malic acid, in particular, has been shown to possess antioxidant properties, helping to neutralize free radicals and reduce oxidative damage. By maintaining a balance between oxidants and antioxidants, these acids help prevent the oxidative damage to lipids, proteins, and DNA, which can contribute to aging, cancer, and other diseases. Citric acid also contributes to antioxidant defense by supporting the generation of NADH and FADH₂, which are involved in electron transfer to mitigate oxidative stress. Together, these acids play a critical role in safeguarding cellular integrity and health.
pH Regulation and Acid-Base Balance
Both malic acid and citric acid have important roles in maintaining acid-base balance within cells and tissues. Malic acid acts as a buffer, helping to stabilize the pH in various cellular compartments by neutralizing excess acids or bases. This is particularly important in the context of cellular metabolism, where fluctuations in pH can significantly affect enzyme activity and metabolic efficiency. Similarly, citric acid, with its acidic nature, helps maintain a stable pH environment in cells, especially within the mitochondria where the TCA cycle takes place. By regulating pH, these acids ensure that cellular processes operate optimally, protecting cells from metabolic disruptions caused by imbalanced acidity or alkalinity.
Biosynthesis and Metabolic Intermediates
In addition to their roles in energy production, malic acid and citric acid serve as important biosynthetic intermediates in various metabolic pathways. Citric acid, as a central metabolite in the TCA cycle, provides carbon skeletons necessary for the synthesis of amino acids, fatty acids, and other key molecules that contribute to cellular growth and repair. Malic acid, through its conversion to oxaloacetate, also provides building blocks for several metabolic pathways, including gluconeogenesis (the production of glucose from non-carbohydrate precursors). Both acids are essential in supporting the anabolic processes in cells, contributing to the formation of molecules required for tissue development, immune response, and overall cellular function. Their roles in biosynthesis extend beyond energy production, ensuring that cells have the necessary components to grow and maintain their structure.
Malic and Citric Acids in Disease Prevention and Management
Malic acid and citric acid contribute to disease prevention and management through their roles in metabolism, antioxidant defense, and cellular function. Their involvement in various biochemical pathways allows them to impact the progression of multiple diseases, including cancer, cardiovascular disease, chronic fatigue, metabolic disorders, and kidney stones.
Cancer Prevention
Both malic acid and citric acid help mitigate oxidative stress, a major factor in cancer development. Their antioxidant properties neutralize reactive oxygen species (ROS), reducing DNA damage and mutagenesis. Citric acid's involvement in the TCA cycle supports energy production, which is essential for DNA repair mechanisms. Research has shown that malic acid can influence the expression of p53, a tumor suppressor gene, which plays a critical role in cell cycle regulation and apoptosis, potentially helping to prevent cancer cell proliferation. Furthermore, citric acid regulates AMPK (AMP-activated protein kinase), a key enzyme in cellular energy homeostasis, and its activation has been linked to the inhibition of cancer cell growth.
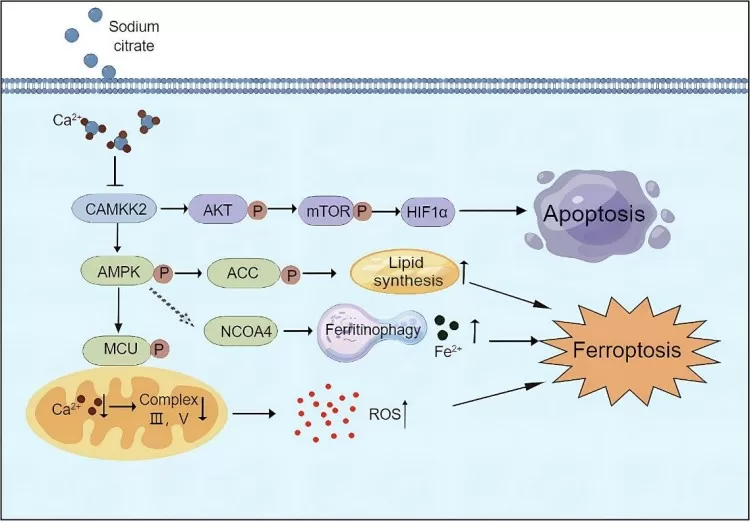
Figure 5. A schematic of the network in which sodium citrate induces apoptosis and ferroptosis in ovarian cancer cells (Wu et al., 2024)
Cardiovascular Health
Both acids contribute to cardiovascular health through their roles in mitochondrial energy production and antioxidative defense. Malic acid has been shown to enhance mitochondrial function, which is vital for the health of heart muscle cells. Citric acid helps maintain optimal pH levels in the heart and regulates NAD+/NADH balance, which impacts cardiac function. Additionally, malic acid's antioxidant effect helps protect blood vessels from oxidative damage, a key factor in the development of atherosclerosis. Research suggests that malate may regulate NO (nitric oxide) production, which plays a critical role in blood vessel dilation and reducing blood pressure, supporting overall cardiovascular function.
Chronic Fatigue Syndrome (CFS) and Fibromyalgia
In CFS and fibromyalgia, mitochondrial dysfunction and impaired energy production are common. Malic acid has shown potential in improving energy metabolism by enhancing ATP production in the mitochondria. Studies have found that malic acid supplementation can help reduce symptoms like fatigue and pain in fibromyalgia patients. Malic acid influences the expression of genes related to mitochondrial biogenesis and cellular energy production, including PGC-1α (peroxisome proliferator-activated receptor-gamma coactivator 1-alpha), a key regulator of mitochondrial function. Citric acid, by supporting TCA cycle activity, also helps increase ATP availability, alleviating energy deficiencies that characterize these chronic conditions.
Metabolic Disorders and Diabetes Management
Malic acid and citric acid both regulate key enzymes and pathways involved in glucose metabolism and insulin sensitivity. Citric acid plays a role in glycolysis, and its presence in the TCA cycle supports efficient glucose oxidation. Research suggests that malic acid can influence the expression of genes involved in lipid metabolism, such as AMPK and SREBP-1c (sterol regulatory element-binding protein 1c), which are critical for improving insulin sensitivity. Malic acid's role in reducing oxidative stress also contributes to diabetes management, as oxidative damage exacerbates insulin resistance. Citric acid helps lower blood glucose levels by promoting efficient glucose utilization in muscle and liver cells.
Kidney Health
Both malic acid and citric acid are beneficial for kidney health, especially in the prevention of kidney stones. Citric acid, with its chelating properties, prevents the formation of calcium oxalate crystals by binding to calcium ions, reducing stone formation. Studies have shown that citrate supplementation can increase urinary citrate levels, which inhibits the crystallization of calcium salts. Malic acid, through its involvement in detoxification processes, helps reduce the burden on kidneys by enhancing the excretion of waste products. It has also been linked to regulating urate levels, which can prevent uric acid stone formation in the kidneys.
Reference
LEHNINGER, A.L.; NELSON, D.L.; COX, M.M. Principles of biochemistry 6. ed. New York: W.H. Freeman and Company, 2013. 1198p.
Koch J, Broeks MH, Gautschi M, Jans J, Laemmle A. Inborn errors of the malate aspartate shuttle - Update on patients and cellular models. Mol Genet Metab. 2024;142(4):108520. doi:10.1016/j.ymgme.2024.108520
Wu Y, Jia C, Liu W, et al. Sodium citrate targeting Ca2+/CAMKK2 pathway exhibits anti-tumor activity through inducing apoptosis and ferroptosis in ovarian cancer. J Adv Res. 2024;65:89-104. doi:10.1016/j.jare.2024.04.033
Read more
- Fumaric Acid Unveiled: From Nature's Palette to Therapeutic Potential
- Pyruvic Acid: A Key Player in Cellular Metabolism and Health
- Lactic Acid: Key Roles in Human Metabolism, Diseases, and Health Implications
- Cholic Acid: The Essential Bile Acid Impacting Digestion and Health
- Multi-Omics Association Analysis Series
- Omics Data Processing Series
- Omics Data Analysis Series


