Unlocking the Power of MALDI-TOF Mass Spectrometry: Principles, Applications, and Future Innovations
Introduction to MALDI-TOF Mass Spectrometry
Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) has emerged as a revolutionary analytical tool, enabling precise analysis of biomolecules with minimal sample degradation. By combining soft ionization techniques with high-resolution mass detection, this technology achieves exceptional sensitivity (detection limits as low as femtomolar levels), high throughput (rapid analysis of multiple samples), and broad mass range capabilities (100 Da to 980 kDa). Its versatility spans biomedical research, chemical analysis, and material science, making it indispensable for modern laboratories. This article delves into the principles, workflows, applications, and limitations of MALDI-TOF MS, providing a holistic understanding of this cutting-edge technology.
MALDI-TOF MS integrates two core components: Matrix-Assisted Laser Desorption/Ionization (MALDI) and a Time-of-Flight (TOF) mass analyzer. The process begins with co-crystallizing the target sample with a light-absorbing matrix. A pulsed laser then irradiates the mixture, triggering desorption and ionization of the sample molecules. The resulting ions are accelerated through an electric field, and their time-of-flight to the detector is measured to determine mass-to-charge ratios (m/z). This method preserves molecular integrity while delivering high-resolution data, making it ideal for analyzing proteins, peptides, oligonucleotides, and metabolites.
How MALDI-TOF MS Works: A Step-by-Step Breakdown
The workflow of MALDI-TOF MS can be divided into two key phases:
1) Matrix-Assisted Laser Desorption/Ionization (MALDI)
- Sample Preparation: The analyte is mixed with a matrix (e.g., α-cyano-4-hydroxycinnamic acid [CHCA] or 2,5-dihydroxybenzoic acid [DHB]). The matrix absorbs laser energy, facilitating efficient ionization of the sample.
- Laser Irradiation: A nitrogen or Nd:YAG laser (typically 337 nm wavelength) pulses onto the sample-matrix mixture. The matrix sublimates, transferring energy to the analyte molecules and inducing ionization.
- Ion Formation: Charged ions (e.g., protonated or deprotonated molecules) are generated, with minimal fragmentation due to the “soft” ionization process.
Key Roles of the Matrix:
- Absorbs laser energy to prevent sample degradation.
- Enhances ionization efficiency through co-crystallization.
- Reduces intermolecular interactions, ensuring clear spectral signals.
2) Time-of-Flight (TOF) Mass Analysis
- Ion Acceleration: Ions are accelerated through an electric field. Lighter ions achieve higher velocities than heavier ones.
- Flight Path: Ions travel through a vacuum flight tube. Their flight time correlates with ‘m/z’ values—larger ions take longer to reach the detector.
- Detection and Data Acquisition: A microchannel plate (MCP) detector records ion arrival times and signal intensities, generating a mass spectrum for analysis.
Case Study: Mapping Metabolites in Strawberry Development
To illustrate MALDI-TOF MS in action, consider a study mapping metabolites during strawberry maturation:
1. Sample Collection: Strawberries at four developmental stages were harvested:
- Green: Immature
- White: Early maturation
- Pink: Intermediate maturation
- Red: Fully mature
2. Tissue Preparation:
- Freezing: Samples were flash-frozen to preserve structural integrity.
- Sectioning: Thin cross-sections were cut using a cryostat.
- Matrix Application: A MALDI matrix (e.g., DHB) was uniformly sprayed onto the sections.
3. Imaging and Analysis:
- Laser Scanning: A rasterized laser scanned the tissue, generating spatially resolved ion signals.
- Data Acquisition: Mass spectra were collected across the tissue surface.
- Spatial Mapping: Software reconstructed molecular distribution maps (e.g., sugars, phenolics) to track metabolic changes during ripening.
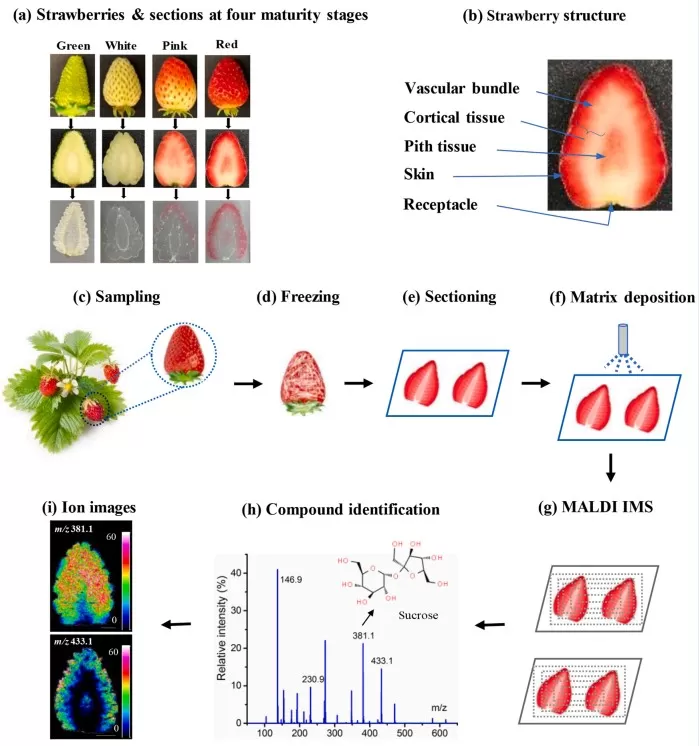
MALDI-TOF imaging reveals metabolite distribution in strawberry
Key Applications of MALDI-TOF MS in Research and Industry
MALDI-TOF MS has revolutionized multiple fields through its unique capabilities:
1) Protein Characterization and Proteomics
- Intact Mass Analysis: Accurately determines protein molecular weights (error ≤500 ppm), validating sequence integrity and detecting post-translational modifications (e.g., glycosylation).
- Peptide Mass Fingerprinting (PMF): Enzymatic digests (e.g., trypsin) generate peptide fragments, which are matched against databases for protein identification. A minimum of four peptide matches ensures reliable results.
- Tandem MS (TOF/TOF): Post-source decay (PSD) or collision-induced dissociation (CID) fragments peptides, enabling *de novo* sequencing for unresolved PMF results.
2) Oligonucleotide Quality Control
- Sequence Validation: Rapidly verifies synthetic oligonucleotides (e.g., primers, probes) with ≤0.03% mass error.
- Salt Tolerance: Performs reliably in the presence of PCR buffers or salts, unlike traditional electrophoresis.
- High-Throughput: Processes samples 10x faster than gel-based methods.
3) Spatial Molecular Imaging (MALDI-IMS)
- Tissue Profiling: Maps metabolites, lipids, and proteins in biological tissues at resolutions up to 10 µm.
- Clinical Applications: In oncology, MALDI-IMS identifies drug distribution patterns and biomarkers linked to tumor heterogeneity.
Advantages and Limitations of MALDI-TOF MS
MALDI-TOF MS excels in high sensitivity, structural preservation, high throughput, and broad mass range (100 Da–980 kDa), making it indispensable for biomolecular analysis. However, challenges such as matrix interference, complex sample purification demands, database dependency, and high instrumentation costs necessitate ongoing methodological and technological refinements.
Advantages and Limitations of MALDI-TOF MS
|
Strengths |
Challenges |
|
High Sensitivity: Detects femtomole-level analytes. |
Matrix Interference: Background signals from matrix clusters may obscure low-abundance ions. |
|
Structural Integrity: Soft ionization preserves biomolecular conformation. |
Complex Samples: Heterogeneous mixtures (e.g., crude extracts) require extensive purification. |
|
High Throughput: Parallel analysis of hundreds of samples. |
Database Dependency: Protein identification relies on existing spectral libraries. |
|
Wide Mass Range: Analyzes molecules from 100 Da to 980 kDa. |
|
Recent advancements in MALDI-TOF mass spectrometry are accelerating breakthroughs in precision medicine and synthetic biology. The integration of automated platforms with robotic systems now enables high-throughput screening, significantly enhancing experimental efficiency and reproducibility by minimizing manual intervention. Concurrently, the development of novel matrix-free substrates, such as nanostructured surfaces, addresses longstanding challenges like background noise, thereby improving detection sensitivity and data accuracy. Furthermore, the adoption of multimodal imaging approaches—such as coupling MALDI-TOF with complementary techniques like Raman spectroscopy—unlocks new dimensions of molecular analysis, facilitating comprehensive multi-omics studies that bridge spatial, structural, and functional insights. Together, these innovations are expanding the scope and precision of MALDI-TOF applications, driving transformative progress across biomedical and industrial research.
References
1. Boesl U. Time-of-flight mass spectrometry: Introduction to the basics. Mass Spectrom Rev. 2017 Jan;36(1):86-109. doi: 10.1002/mas.21520.
2. Guerrera IC, Kleiner O. Application of mass spectrometry in proteomics. Biosci Rep. 2005 Feb-Apr;25(1-2):71-93. doi: 10.1007/s10540-005-2849-x.
3. Calvano CD, De Ceglie C, Zambonin CG. Proteomic analysis of complex protein samples by MALDI-TOF mass spectrometry. Methods Mol Biol. 2014;1129:365-80. doi: 10.1007/978-1-62703-977-2_27.
4. Hawkinson TR, Sun RC. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging of Glycogen In Situ. Methods Mol Biol. 2022;2437:215-228. doi: 10.1007/978-1-0716-2030-4_15.
5. Wang J, Yang E, Chaurand P, Raghavan V. Visualizing the distribution of strawberry plant metabolites at different maturity stages by MALDI-TOF imaging mass spectrometry. Food Chem. 2021 May 30;345:128838. doi: 10.1016/j.foodchem.2020.128838.
Read more
- Spatial Metabolomics: Transforming Biomedical and Agricultural Research
- Spatial Metabolomics Explained: How It Works and Its Role in Cancer Research
- MALDI, DESI, or SIMS? How to Choose the Best MSI Techniques for Spatial Metabolomics
- How to Prepare Samples for Spatial Metabolomics: The Essential Guide You Need
- Unlocking Precision in Spatial Metabolomics: Essential Detection Parameters for Cutting-Edge Research


