An Overview of Mainstream Proteomics Techniques
In proteomics research, several mainstream techniques are widely used to analyze and quantify proteins. This article provides an overview of these techniques, focusing on qualitative and quantitative proteomics methods. Here are the main sections of this article:
1. Introduction to Mainstream Proteomics Techniques
2. Label-Free DDA Quantitative Proteomics
3. Advantages and Disadvantages of Label-Free Quantification
4. TMT/iTRAQ Labeled Quantitative Proteomics
5. DIA Quantitative Proteomics
6. Comparison of DDA and DIA Acquisition Modes
Introduction to Mainstream Proteomics Techniques
In mass spectrometry-based proteomics, the primary methods fall into two main categories: qualitative and quantitative proteomics, with the latter being the focus of proteomics research. The most widely used approaches in quantitative proteomics include label-free quantification [1], isobaric labeling techniques such as TMT/iTRAQ quantification [2-4], and data-independent acquisition (DIA) or SWATH methods [5, 6]. Depending on the subject of study, research objectives, or available instruments, various proteomics methods may evolve, but they are all based on these foundational approaches.
1. Based on Different Instruments:
3D label-free proteomics, 3D TMT proteomics, 3D DIA proteomics, 4D label-free proteomics, 4D DIA proteomics, and more others.
2. Based on Different Study Subjects:
Blood proteomics, urine proteomics, metaproteomics, low-abundance proteomics, single-cell proteomics, spatial proteomics, and more others.
3. Based on Different Research Objectives
a) Post-translational modification proteomics: phosphorylationomics, acetylationomics, glycosylationomics, ubiquitinationomics, and more others.
b) Exosome proteomics
c) Organelle proteomics: chloroplast proteomics, mitochondrial proteomics, membrane proteomics, and more.
Label-Free DDA Quantitative Proteomics
Label-free quantitative proteomics is the simplest and earliest method used for quantifying proteomic data. The main experimental steps include sample preparation, protein extraction, protein digestion, (peptide fractionation), MS detection, database searching, and bioinformatics analysis. In early research, due to limitations in mass spectrometer performance, offline fractionation at the peptide level was often employed to enhance the depth of peptide/protein identification. However, this process also introduced higher quantification errors. As mass spectrometer technology has improved, this strategy is now rarely used. Additionally, in label-free quantitative proteomics, MS detection applies data-dependent acquisition (DDA) mode.
To provide a practical example, imagine we have two samples: Sample1 and Sample2. The basic strategy in label-free quantification involves extracting and digesting total proteins from each sample to produce peptides. These peptides are then analyzed separately by mass spectrometry, resulting in two raw data files. Finally, these two files are searched and analyzed together to generate the final qualitative and quantitative results.
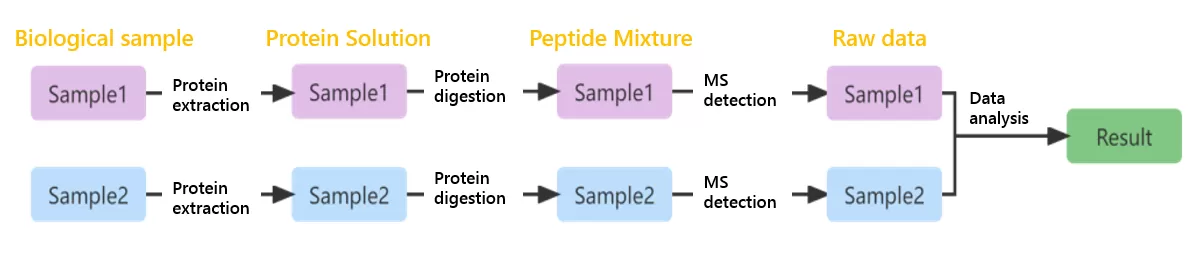
Advantages and Disadvantages of Label-Free Quantification
Advantages:
1. The experimental process is straightforward and cost-effective.
2. Not constrained by sample size; minimal batch effects even with large sample sets make it suitable for high-throughput studies.
3. Offers a broader linear dynamic range.
4. The 4D label-free technology based on timsTOF Pro2 achieves detection depth comparable to traditional TMT.
Disadvantages:
1. Quantitative stability can be significantly influenced by the experimenter.
2. Quantitative accuracy is slightly lower than that of TMT.
3. The detection depth of most mass spectrometers is not as deep as TMT quantification techniques.
4. Has a higher proportion of missing values compared to TMT and DIA methods.
TMT/iTRAQ Labelled Quantitative Proteomics
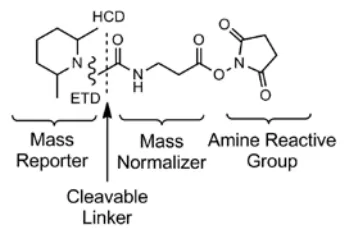 TMT/iTRAQ quantitative proteomics is a leading method in isobaric labeling, leveraging the principles of isotope labeling. It uses different isotope tags to label peptides at specific amino acid sites, enabling differentiation of peptides from various sources. These reagents consist of three main components: the reporter group, balance group, and reactive group. In a specific kit, these three groups use different combinations of C13 and N15 isotopes to maintain a consistent molecular weight across all reagents. The reporter group varies in molecular weight, allowing for the distinction of peptide origins after labeling. iTRAQ kits include iTRAQ4-plex and iTRAQ8-plex, while TMT kits include TMT6-plex, TMT10-plex, TMT11-plex, TMT Pro 16-plex, and TMTPro 18-plex. This article uses TMT10-plex as an example to describe its basic principles and experimental workflow.
TMT/iTRAQ quantitative proteomics is a leading method in isobaric labeling, leveraging the principles of isotope labeling. It uses different isotope tags to label peptides at specific amino acid sites, enabling differentiation of peptides from various sources. These reagents consist of three main components: the reporter group, balance group, and reactive group. In a specific kit, these three groups use different combinations of C13 and N15 isotopes to maintain a consistent molecular weight across all reagents. The reporter group varies in molecular weight, allowing for the distinction of peptide origins after labeling. iTRAQ kits include iTRAQ4-plex and iTRAQ8-plex, while TMT kits include TMT6-plex, TMT10-plex, TMT11-plex, TMT Pro 16-plex, and TMTPro 18-plex. This article uses TMT10-plex as an example to describe its basic principles and experimental workflow.
The molecular structure of TMT10-plex is identical to that of TMT6-plex (as shown in Figure 2), with the molecular formula H(20)C(8)13C(4)N15NO(2). The differences between them are in the number and positions of 13C and 15N isotopes. Table 1 lists the molecular weights of the reporter groups produced under ETD and HCD fragmentation modes. It shows that TMT10-plex consists of 10 reagents with identical molecular structures but different molecular weights in their reporter groups.
Table 1. The molecular weight of the reporter group in TMT10-plex
|
Labelling Reagent |
Reporter Group Molecular Weight (HCD) |
Reporter Group Molecular Weight (ETD) |
|
126 |
126.127726 |
114.127725 |
|
127N |
127.124761 |
115.124760 |
|
127C |
127.131081 |
114.127725 |
|
128N |
128.128116 |
115.124760 |
|
128C |
128.134436 |
116.134433 |
|
129N |
129.131471 |
117.131468 |
|
129C |
129.137790 |
116.134433 |
|
130N |
130.134825 |
117.131468 |
|
130C |
130.141145 |
118.141141 |
|
131 |
131.138180 |
119.138176 |
The core process involves reacting and labeling different samples with distinct reagents, tagging all peptides in a specific sample with a particular labeling reagent. Once all samples are labeled, equal amounts of peptides from each sample are mixed and fragtionation offline before undergoing mass spectrometry analysis. During mass spectrometry, since the total molecular weight of all reagents is the same, the precursor ions of the same peptide from different samples present as a single peak, representing the combined signal from multiple samples for that peptide. This enhances the selection of precursor peptide ions for fragmentation. When the precursor peptide ion is selected and fragmented, the reporter groups of the labeling reagents from different samples also fragment, releasing free ions that are detected by the mass spectrometer. Ultimately, the mass spectrometry data from the reporter groups enables the quantification of the same peptides across different samples.
Advantages of TMT/iTRAQ Quantitation:
1. Multiplexed detection minimizes errors and improves quantification accuracy.
2. Up to 18 samples can be simultaneously labeled in one experiment.
3. Offline fractionation of mixed peptides increases the depth of detection.
Disadvantages of TMT/iTRAQ Quantitation:
1. Limited sample numbers in each batch and batch effects exist among batches.
2. Compression effects can result in underestimation of quantification.
3. Unable to determine the presence or absence of peptides.
DIA Quantitative Proteomics
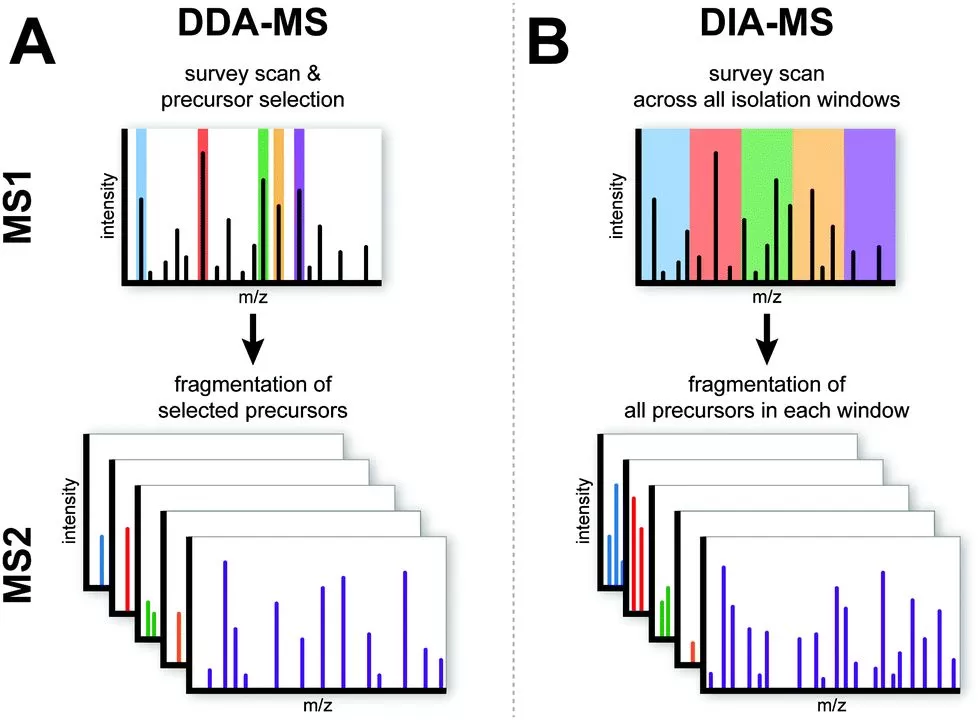 DIA, short for Data Independent Acquisition, is a cutting-edge mass spectrometry acquisition technique. Traditional mass spectrometry methods typically employ DDA, or data-dependent acquisition, such as label-free quantification and TMT/iTRAQ quantification. In DDA, peptide precursor ions are selectively fragmented (choosing 10-30 in descending order of intensity), whereas in DIA, all peptide precursor ions are fragmented, capturing a broader range of data. As a result, DIA provides more comprehensive data, while DDA may lose most peptide information. Given that DIA fragments all peptide precursor ions into shorter secondary fragment ions, it can accurately recreate the chromatogram of nanoUHPLC at the fragment ion level, allowing DIA quantification to integrate fragment ions over time to estimate peptide abundance. Quantification using secondary fragment ions is inherently more precise than using primary ions (as in DDA) and exhibits smaller random errors. Essentially, DIA is a form of label-free quantification.
DIA, short for Data Independent Acquisition, is a cutting-edge mass spectrometry acquisition technique. Traditional mass spectrometry methods typically employ DDA, or data-dependent acquisition, such as label-free quantification and TMT/iTRAQ quantification. In DDA, peptide precursor ions are selectively fragmented (choosing 10-30 in descending order of intensity), whereas in DIA, all peptide precursor ions are fragmented, capturing a broader range of data. As a result, DIA provides more comprehensive data, while DDA may lose most peptide information. Given that DIA fragments all peptide precursor ions into shorter secondary fragment ions, it can accurately recreate the chromatogram of nanoUHPLC at the fragment ion level, allowing DIA quantification to integrate fragment ions over time to estimate peptide abundance. Quantification using secondary fragment ions is inherently more precise than using primary ions (as in DDA) and exhibits smaller random errors. Essentially, DIA is a form of label-free quantification.
Figure 3A shows the DDA acquisition mode. In this approach, after an MS1 full scan, a select number of high-intensity precursor ions are chosen for subsequent MS2 scans, where they are individually selected and fragmented into secondary ions. Figure 3B illustrates the DIA acquisition mode. In this method, following an MS1 full scan, the entire mass-to-charge ratio range of MS1 is divided into multiple windows, each containing a variety of peptide precursor ions. In the subsequent MS2 scans, all the peptide precursor ions within each window are simultaneously fragmented.
The DIA acquisition mode fragments all peptide precursor ions based on mass-to-charge ratio windows, rather than selecting them by ion intensity. This approach yields more comprehensive data, resulting in fewer missing values and less variability in the final quantification results. Additionally, because DIA quantification relies on MS2 fragment ion information, it offers greater accuracy compared to label-free DDA quantification.
Comparison of DDA and DIA Acquisition Modes
In DDA, a single MS2 spectrum theoretically represents the fragment ions of only one peptide precursor ion, whereas in DIA, a single MS2 spectrum theoretically contains fragment ions from multiple peptide precursor ions. As a result, DIA data is more complex than DDA data, and data analysis cannot simply rely on comparing theoretical protein sequence databases with MS2 spectra as in DDA. In DIA data analysis, researchers often first use the DDA mode to construct a spectral library containing peptide sequences, retention times, fragment ion mass-to-charge ratios, and ion intensity information. Then, by comparing the fragment ions from the spectral library with those in the DIA collected data, the peptide sequences present in a particular MS2 spectrum can be identified to enable peptide identification. Utilizing the completeness of DIA data, extracted ion chromatograms (XICs) are built from the fragment ions, and peak areas are calculated. Peptide peak areas can be inferred from fragment ion peak areas, thus enabling peptide quantification.
Notably, with advancements in instrument technology (especially with the introduction of the timsTOF Pro series of instruments based on ion mobility separation) and the application of deep learning and machine learning algorithms, DIA data analysis is moving toward using DIA data directly with protein sequences to achieve peptide and protein identification and quantification without the need for a spectral library.
By the end of this article, you'll have a comprehensive understanding of the primary methods in proteomics and their respective strengths and weaknesses, helping you choose the optimal technique for your research.
Read more:
· A Guide to Protein Database Selection
· MetwareBio Launches Proteomics Services
· What is Isoelectric Points of Amino Acids: Essential Calculations and Practical Applications
· Comparison and Application of Proteomic Technologies
· Demystifying Proteomics Research Strategies and Content in a Single Read
· Optimal Protein Database Selection: Insights from Experimental Data
· Protein sample preparation tips: Serum or Plasma?
Referfences
1. Neilson, K.A., et al., Less label, more free: approaches in label-free quantitative mass spectrometry. Proteomics, 2011. 11(4): p. 535-53.
2. Choe, L., et al., 8-plex quantitation of changes in cerebrospinal fluid protein expression in subjects undergoing intravenous immunoglobulin treatment for Alzheimer's disease. Proteomics, 2007. 7(20): p. 3651-60.
3. Ross, P.L., et al., Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics, 2004. 3(12): p. 1154-69.
4. Thompson, A., et al., Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem, 2003. 75(8): p. 1895-904.
5. Gillet, L.C., et al., Targeted data extraction of the MS/MS spectra generated by data-independent acquisition: a new concept for consistent and accurate proteome analysis. Mol Cell Proteomics, 2012. 11(6): p. O111 016717.
6. Sidoli, S., R. Fujiwara, and B.A. Garcia, Multiplexed data independent acquisition (MSX-DIA) applied by high resolution mass spectrometry improves quantification quality for the analysis of histone peptides. Proteomics, 2016. 16(15-16): p. 2095-105.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


