Lipidomics Demystified: Exploring Lipid Classification, Structures, Functions, and Analytical Techniques
Lipids are essential biomolecules that play pivotal roles in energy storage, cellular structure, and signaling. Their complexity and diversity have inspired the field of lipidomics, a specialized branch of metabolomics that deciphers lipid functions and mechanisms in health and disease. From understanding lipid classification and functions to exploring advanced analytical techniques like LC-MS and NMR, this guide provides a comprehensive overview of lipid science, its biological significance, and state-of-the-art methods for lipid analysis.
- Lipids: Classification, Structure, and Complexity
- The Many Roles of Lipids: Beyond Membranes and Energy
- What is Lipidomics? Decoding the Science of Lipid Analysis
- Lipidomics VS Metabolomics: What’s the Difference?
- Cutting-Edge Lipidomics Analysis Methods
- The Lipidomics Workflow: From Sample to Insight
Lipids: Classification, Structure, and Complexity
Lipids are a diverse group of hydrophobic or amphipathic organic molecules that are insoluble in water but soluble in non-polar solvents. They are essential to the structure and function of living organisms, playing key roles in energy storage, membrane structure, and signaling. To promote international collaboration in lipid research, the LIPID Metabolites and Pathways Strategy (LIPID MAPS) established a comprehensive lipid classification platform in 2005. This platform was designed to facilitate the informatics analysis of vast lipid datasets. Lipids were classified into eight main categories: fatty acyls, glycerolipids, glycerophospholipids, sphingolipids, sterol lipids, prenol lipids, saccharolipids, and polyketides. Our quantitative lipidomics services enable precise measurement of sphingolipids, phospholipids, and more.
Lipid categories and examples
|
Category |
Abbreviation |
Example |
|
Fatty acyls |
FA |
dodecanoic acid |
|
Glycerolipids |
GL |
l-hexadecanoyl-2-(9Z-octadecenoyl)-sn-glycerol |
|
Glycerophospholipids |
GP |
l-hexadecanoyl-2-(9Zoctadecenoyl)-sn-glycero-3-phosphocholine |
|
SP |
N-(tetradecanoyl)-sphing-4-enine |
|
|
ST |
cholest-5-en-3β-ol |
|
|
Prenol lipids |
PR |
2E,6E-farnesol |
|
Saccharolipids |
SL |
UDP-3-O-(3R-hydroxy-tetradecanoyl)- αD-Nacetylglucosamine |
|
Polyketides |
PK |
aflatoxin B₁ |
Fatty Acyls
Fatty acyls (FA) are a diverse class of molecules primarily synthesized through the chain elongation of acetyl-CoA with malonyl-CoA or methylmalonyl-CoA. These molecules form the fundamental building blocks of more complex lipids, with their hydrophobic nature arising from repeated methylene groups in their structure. Key subclasses include free fatty acids, eicosanoids, and Fatty esters.
Glycerolipids
Glycerolipids primarily consist of mono-, di-, and tri-substituted glycerols, with triacylglycerols (triglycerides) being the most recognized members. These are the main storage fats in mammalian tissues. Additionally, macrocyclic ether lipids, found in archaebacterial membranes, are categorized under glycerolipids.
Glycerophospholipids
Also known as phospholipids, glycerophospholipids are essential components of cell membranes and play critical roles in signaling and metabolism. Their structure is defined by the polar head group attached to the glycerol backbone, varying based on the organism. Common examples include phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidylserine (PS).
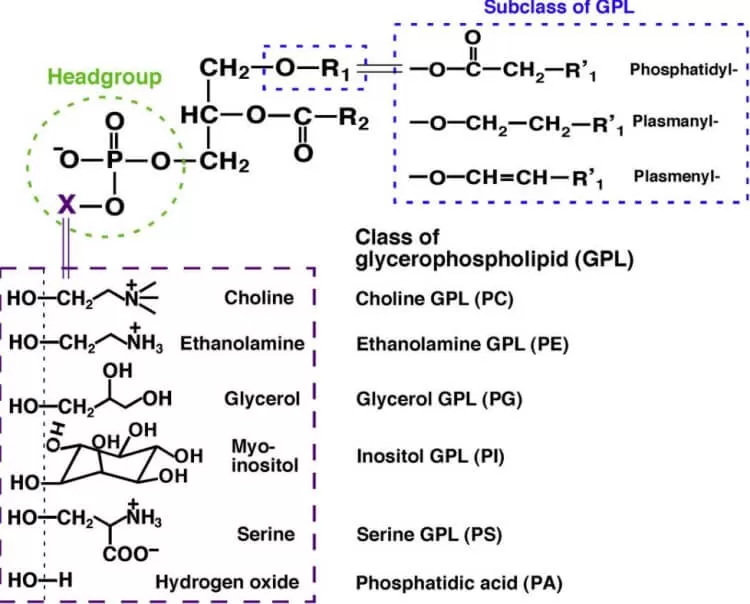
Summary of classes, subclasses, and molecular species in glycerophospholipid
Sphingolipids
Sphingolipids are a structurally complex group of lipids derived from a sphingoid base backbone. They are synthesized from serine and a long-chain fatty acyl-CoA, resulting in ceramides, phosphosphingolipids, glycosphingolipids, and other derivatives. Subclasses include sphingoid bases, amide-linked fatty acid derivatives like ceramides, and glycosphingolipids such as cerebrosides and gangliosides. They are vital in cell structure and signaling.
Sterol Lipids
Sterol lipids, such as cholesterol and its derivatives, are crucial components of cellular membranes. Steroids, characterized by a four-ring core structure, serve diverse functions as hormones and signaling molecules. This category includes C18 (estrogens), C19 (androgens like testosterone), C21 (progestogens and corticosteroids), and secosteroids (vitamin D). Bile acids and conjugates, synthesized from cholesterol, are also part of this group.
Prenol Lipids
Prenol lipids are derived from the isoprenoid precursors isopentenyl diphosphate and dimethylallyl diphosphate via the mevalonic acid pathway. These lipids, including polyterpenes (structures with more than 40 carbons) and carotenoids, play roles as antioxidants and vitamin A precursors. Other significant classes include ubiquinones and vitamins E and K, which combine isoprenoid tails with quinonoid cores.
Saccharolipids
Saccharolipids feature fatty acids linked directly to sugar backbones, enabling compatibility with membrane bilayers. This unique structure replaces the glycerol backbone typically found in glycerolipids and glycerophospholipids, expanding the functional versatility of these lipids.
Polyketides
Polyketides are synthesized through the polymerization of acetyl and propionyl units by enzymatic systems resembling fatty acid synthases. Known for their structural diversity, they include many secondary metabolites and natural products. Polyketides often undergo modifications such as glycosylation, methylation, or hydroxylation, yielding compounds like erythromycins, tetracyclines, and antitumor agents like epothilones. These compounds are vital for pharmaceutical applications due to their antimicrobial, antiparasitic, and anticancer properties.
The Many Roles of Lipids: Beyond Membranes and Energy
Lipids are far more than passive biomolecules. They are dynamic entities driving cellular architecture, energy balance, communication, and disease processes, underscoring their multifaceted importance in life sciences.
1. Membrane Composition and Fluidity
Lipids are the cornerstone of cellular membranes, forming lipid bilayers that provide structural integrity and compartmentalization within cells. The amphipathic nature of phospholipids and sphingolipids, with hydrophilic heads and hydrophobic tails, enables the formation of this bilayer. Membrane fluidity, critical for protein mobility, endocytosis, and cellular signaling, is regulated by lipid composition. Cholesterol, for instance, modulates membrane rigidity by filling gaps between unsaturated fatty acids while preventing excessive rigidity under colder conditions. This balance is essential for maintaining membrane function across varying physiological states.
2. Energy Storage
Lipids, primarily in the form of triacylglycerols (triglycerides), serve as highly efficient energy reservoirs. They provide more than double the caloric content per gram compared to carbohydrates or proteins. Stored in adipose tissue, lipids offer long-term energy reserves, critical during fasting or extended physical activity. Furthermore, lipids act as thermal insulators, preserving body heat in cold conditions, and their hydrophobic nature ensures efficient, compact energy storage without water weight.
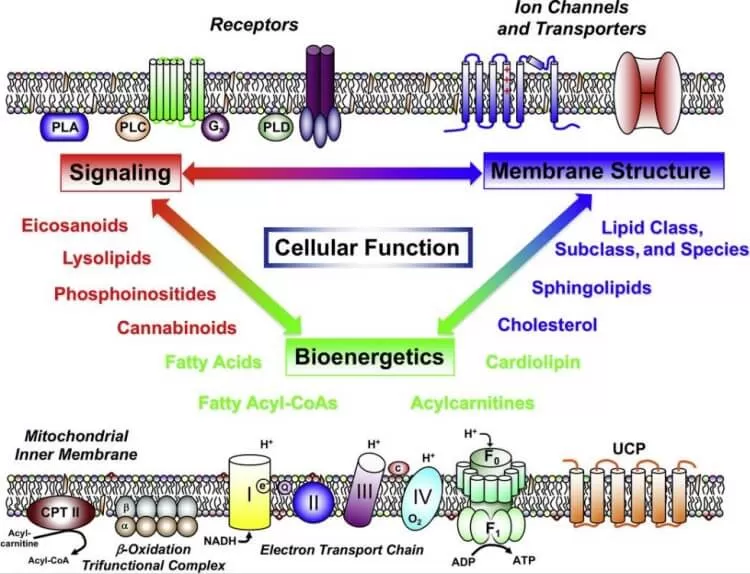
The pleiotropic roles of lipids in cellular functions
3. Signaling and Regulation
Lipids function as dynamic signaling molecules influencing various cellular processes. Phosphoinositides (e.g., PI3K pathway lipids) regulate intracellular signaling cascades, controlling cell proliferation, apoptosis, and differentiation. Eicosanoids, derived from arachidonic acid, are involved in inflammation, immune responses, and pain regulation. Similarly, steroid hormones like estrogens and androgens, derived from cholesterol, act as long-range chemical messengers, coordinating processes such as reproduction and stress response.
4. Cellular Communication
Lipids contribute to intercellular communication through exosomes and lipid rafts. Exosomes, small extracellular vesicles rich in lipids like sphingomyelins and cholesterol, transport proteins, RNA, and bioactive lipids between cells, playing roles in immune modulation and cancer metastasis. Lipid rafts, microdomains within membranes, organize receptor clustering and signal transduction, ensuring precise cellular communication. Glycosphingolipids also facilitate cell recognition and adhesion, fundamental in immune responses and tissue integrity.
5. Disease Implications
Dysregulated lipid metabolism is implicated in various diseases. Excess lipid accumulation leads to obesity and associated conditions like type 2 diabetes and cardiovascular diseases. Altered lipid signaling contributes to chronic inflammation, a hallmark of metabolic syndromes. Sphingolipids and ceramides are implicated in neurodegenerative disorders such as Alzheimer’s, where they influence amyloid-beta aggregation. Additionally, abnormal cholesterol metabolism contributes to atherosclerosis, while altered phospholipid signaling is linked to cancer progression. Understanding these mechanisms highlights lipids as potential therapeutic targets.
What is Lipidomics? Decoding the Science of Lipid Analysis
Lipidomics is a specialized branch of metabolomics dedicated to the in-depth study of lipids in biological systems. It aims to profile, identify, and quantify lipid species while uncovering their roles in cellular processes, physiology, and pathology. Lipidomics helps scientists explore lipid-related mechanisms in metabolism, signaling, and disease states such as diabetes, cancer, and neurodegenerative disorders.With advancements in analytical technologies like mass spectrometry (MS) and bioinformatics, lipidomics has evolved into a powerful tool for translational medicine, nutritional science, and drug discovery.
Lipidomics VS Metabolomics: What’s the Difference?
Metabolomics and lipidomics are complementary omics technologies, each offering unique insights into biological systems. Metabolomics provides a broad-spectrum analysis of the metabolome, encompassing diverse small molecules such as amino acids, carbohydrates, nucleotides, and lipids. It aims to understand entire metabolic pathways and their connection to phenotypes. In contrast, lipidomics is a subfield of metabolomics specializing in the lipidome, delving deeper into lipid-related processes, including membrane dynamics, signaling, and energy metabolism, making it particularly relevant in studies of metabolic, cardiovascular, and neurodegenerative diseases.
While metabolomics offers a systems-wide perspective, lipidomics provides detailed insights into lipid-specific pathways, making them highly synergistic. For instance, in diabetes research, metabolomics may highlight glucose metabolism disruptions, whereas lipidomics could focus on lipid dysregulation. Together, they provide a comprehensive toolkit for studying disease mechanisms, identifying biomarkers, and understanding the interplay of metabolism and cellular functions. Their integration enables a more thorough understanding of health and disease, paving the way for personalized medicine and therapeutic innovations.
Cutting-Edge Lipidomics Analysis Methods
Lipidomics employs advanced technologies to identify, quantify, and profile lipids in biological samples. By providing insights into lipid metabolism and its roles in health and disease, lipidomics bridges various research areas, including metabolomics, cell biology, and biochemistry. Below is a comprehensive introduction to state-of-the-art lipidomics methods, highlighting their principles, applications, and advantages.
1. Liquid Chromatography-Mass Spectrometry (LC-MS)
Mass spectrometry (MS) is the cornerstone of lipidomics, offering unparalleled sensitivity and specificity in lipid analysis. It enables both targeted and untargeted approaches for comprehensive lipid profiling. High-Resolution MS instruments provide accurate mass measurements, enabling precise identification of lipid species. MS/MS enhances structural elucidation by fragmenting ions to reveal detailed molecular features such as chain length, unsaturation, and functional groups. It is critical for distinguishing isobaric lipids. Combining chromatography with MS enhances lipid separation and detection, especially in complex biological matrices.
2. Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR provides structural information about lipids without requiring ionization or derivatization. It offers non-destructive analysis and is ideal for quantifying lipid concentrations. It complements MS-based lipidomics by providing detailed stereochemical information. But lower sensitivity compared to MS restricts its application to less complex samples or targeted lipid analysis.
3. Imaging Mass Spectrometry (IMS)
IMS visualizes lipid distributions within tissues, revealing spatial lipidomics. Matrix-Assisted Laser Desorption Ionization (MALDI)-IMS and Desorption Electrospray Ionization (DESI) IMS allow for label-free lipid imaging in tissue sections. This method is invaluable for understanding lipid roles in tissue organization, such as tumor microenvironments.IMS is used in neurobiology to study brain lipid distributions and in oncology for tumor diagnostics.
The Lipidomics Workflow: From Sample to Insight
The Lipidomics workflow is a meticulously designed process to profile and quantify lipid species in biological samples. This workflow integrates experimental design, sample preparation, lipid extraction, analysis, and data interpretation to ensure high-quality results.
1. Sample Collection
Proper sample collection is crucial for reliable lipidomics, as lipid profiles are highly sensitive to environmental and handling conditions. Biological samples such as blood, plasma, serum, tissues, or cell cultures must be collected promptly and under controlled conditions to prevent lipid degradation or alteration. Using antioxidants or inhibitors during collection can minimize oxidation and enzymatic activity that might interfere with lipid integrity. Samples should be rapidly processed and stored at low temperatures (e.g., -80°C) to preserve lipid stability. Standardized protocols are essential to reduce variability, ensuring reproducibility and comparability of lipidomic data across studies. Careful handling during this initial step lays the groundwork for accurate downstream analyses.
2. Lipid Extraction
Efficient lipid extraction is essential for achieving accurate and reproducible lipid profiling. Common methods include the Bligh and Dyer method, which uses a biphasic system of chloroform and methanol to separate lipids into the organic phase; the Folch method, a similar approach optimized for specific sample types like tissues; and the MTBE method, a safer and more efficient alternative that avoids chloroform. Preparation steps involve adding internal standards to facilitate quantification, drying the extracts under nitrogen or vacuum to concentrate the lipids, and reconstituting them in solvents suitable for the analytical method, such as mass spectrometry (MS). These steps ensure that lipid samples are well-prepared for precise and reliable analysis.
3. Lipid Detection
Lipid detection is a critical step in lipidomics, relying on advanced analytical techniques to accurately identify and quantify lipid molecules. Mass spectrometry (MS), often coupled with liquid chromatography (LC-MS), is the most widely used method, providing high sensitivity and resolution for separating complex lipid mixtures and detecting their molecular species. Techniques like tandem mass spectrometry (MS/MS) allow for detailed structural elucidation, including chain length, saturation, and functional groups. Complementary methods such as nuclear magnetic resonance (NMR) can be employed for detailed structural insights, while infrared spectroscopy and gas chromatography (GC) are valuable for specific lipid classes like fatty acids. Robust internal standards and carefully optimized workflows ensure that detection processes are both accurate and reproducible, supporting the identification of diverse lipids in biological samples.
4. Data Analysis
The immense complexity of lipid data demands sophisticated computational tools for accurate processing and identification. Software such as LipidSearch, LIPID MAPS, and MS-DIAL enables the annotation of lipid species by matching experimental data with extensive reference libraries. Additionally, platforms like MetaboAnalyst support statistical analysis, pathway mapping, and biomarker discovery. Quantification strategies in lipidomics include relative quantification, which compares signal intensities across samples, and absolute quantification, which employs internal standards. The use of isotope-labeled standards further enhances the precision and accuracy of absolute quantification, ensuring reliable data for downstream applications.
Need lipid class–specific profiling for your research? Learn more about our lipidomics solutions.
Reference:
Han X, Gross R W. The foundations and development of lipidomics[J]. Journal of lipid research, 2022: 100164. DOI: 10.1016/j.jlr.2021.100164
Fahy E, Subramaniam S, Brown H A, et al. A comprehensive classification system for lipids1[J]. Journal of lipid research, 2005, 46(5): 839-861. DOI: 10.1194/jlr.E400004-JLR200
Read more
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


