Unveiling Leukemia's Metabolic Secrets: How Glucose Inhibition Exposes Mitochondrial Dependence
We are excited to share a groundbreaking study published in Cell Communication and Signaling, where researchers explored the metabolic adaptations of leukemia cells under glucose uptake inhibition. Utilizing energy metabolism profiling and advanced bioenergetic profiling, the study reveals how acute glucose deprivation forces leukemia cells to rely heavily on mitochondrial respiration for survival. This research highlights the critical role of metabolic reprogramming in cancer cell survival and offers new therapeutic insights for targeting leukemia, particularly in cases driven by oncogenic RAS mutations. MetwareBio's state-of-the-art metabolomics platform was instrumental in characterizing a wide range of metabolites, facilitating in-depth and precise understanding of disease mechanisms.
Targeting Glucose Uptake Inhibition in AML: Unveiling Mitochondrial Respiration Dependency
Acute myeloid leukemia (AML) is a devastating blood cancer characterized by rapid proliferation and metabolic dysregulation. One hallmark of cancer is the increased reliance on glucose metabolism to fuel growth and survival, a phenomenon known as the Warburg effect. However, the metabolic plasticity of cancer cells allows them to adapt to glucose deprivation, often shifting to alternative energy sources. This study investigates how leukemia cells respond to acute glucose uptake inhibition using a potent small molecule inhibitor, KL-11743, and uncovers a critical dependency on mitochondrial respiration. By employing energy metabolism profiling and bioenergetic profiling, the researchers provide new insights into the metabolic vulnerabilities of leukemia cells, paving the way for novel therapeutic strategies.
KL-11743 Effectively Inhibits Glucose Uptake and Induces Mitochondrial Respiration
The study began by evaluating the effects of KL-11743, a Class I glucose transporter inhibitor, on leukemia cell lines. Using glucose uptake assays and extracellular flux analysis, the researchers demonstrated that KL-11743 significantly reduced glucose consumption and glycolysis. Surprisingly, this inhibition did not lead to immediate cell death. Instead, the cells compensated by increasing mitochondrial oxidative phosphorylation (OXPHOS), as evidenced by elevated oxygen consumption rates (OCR). This metabolic shift was particularly pronounced in cells with high glycolytic activity, such as MOLT-4 and NB4. The findings suggest that leukemia cells can adapt to glucose deprivation by enhancing mitochondrial respiration, highlighting the importance of targeting both glycolysis and mitochondrial metabolism in cancer therapy.
Glutamine Metabolism Plays a Key Role in Metabolic Reprogramming
To further understand the metabolic adaptations, the researchers performed energy metabolism profiling on KL-11743-treated cells. They observed significant changes in glutamine and glutamate metabolism, with increased export of glutamate suggesting a rerouting of glutamine metabolism. When glutamine was withdrawn, the KL-11743-induced increase in mitochondrial respiration was severely blunted, indicating that glutamine is essential for maintaining the bioenergetic shift. This finding underscores the dual dependency of leukemia cells on both glucose and glutamine metabolism, providing a potential target for combination therapies.
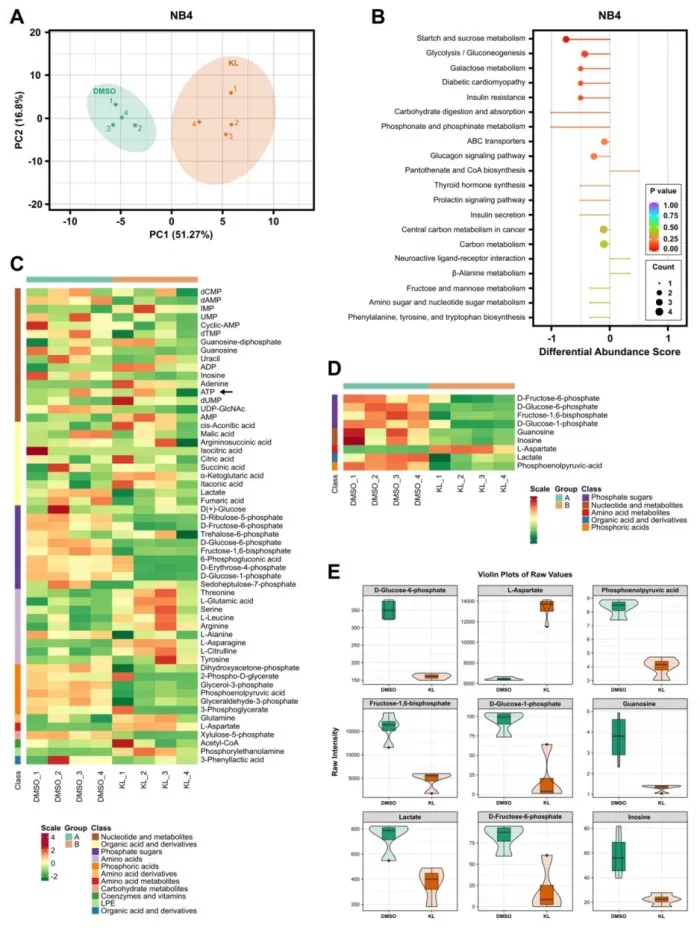
Targeted metabolomics of NB4 treated with KL-11743
Complex I Inhibition Synergizes with Glucose Uptake Blockade to Induce Cell Death
The study also explored the role of mitochondrial Complex I (CI) in the metabolic adaptation to glucose deprivation. Using in-gel activity assays and CI-specific OCR measurements, the researchers found that KL-11743 treatment increased CI activity and assembly in certain leukemia cell lines. When combined with IACS-010759, a clinically relevant CI inhibitor, KL-11743 induced synthetic lethality, leading to significant cell death. This synergistic effect was particularly strong in AML patient-derived cells with oncogenic RAS mutations, suggesting that dual inhibition of glucose uptake and mitochondrial respiration could be a promising therapeutic strategy for RAS-driven leukemias.
Targeting Leukemia Metabolism: Mitochondrial Adaptation and Therapeutic Potential
This study provides compelling evidence that leukemia cells can adapt to glucose deprivation by enhancing mitochondrial respiration, a process driven by glutamine metabolism and increased Complex I activity. The use of energy metabolism profiling and bioenergetic profiling was instrumental in uncovering these metabolic adaptations, demonstrating the power of multi-omics approaches in cancer research. The findings also highlight the therapeutic potential of combining glucose uptake inhibitors with mitochondrial respiration blockers, particularly in RAS-mutated leukemias.
The research aligns with emerging trends in cancer metabolism, where targeting metabolic vulnerabilities is becoming a key strategy for overcoming drug resistance. However, the study also raises important questions about the broader applicability of this approach. For instance, how do other cancer types with different metabolic profiles respond to similar treatments? Additionally, the potential toxicity of mitochondrial inhibitors in non-cancer cells remains a concern, as seen in clinical trials of IACS-010759. Future studies should explore these challenges and investigate the long-term effects of metabolic inhibition in vivo.
By leveraging the latest in metabolomics and bioenergetic analysis, this study not only advances our understanding of leukemia metabolism but also opens new avenues for therapeutic intervention. Stay tuned for more insights from the world of metabolomics!
Enhance Your Research through MetwareBio’s Specialized Expertise
At MetwareBio, we specialize in innovative proteomcis, metabolomics and multi-omics technologies to life science and health research. Our advanced mass spectrometry technologies and expert team ensure high-quality, reproducible results, enabling groundbreaking discoveries like those in this study. Whether you're studying cancer metabolism, plant biology, or drug development, our one-stop metabolomics services can help you achieve your research goals.
Reference
Komza, M., Khatun, J., Gelles, J.D. et al. Metabolic adaptations to acute glucose uptake inhibition converge upon mitochondrial respiration for leukemia cell survival. Cell Commun Signal 23, 47 (2025). https://doi.org/10.1186/s12964-025-02044-y
Read more
- Unveiling Therapeutic Targets for Liver Damage after Liver Transplantation: Gp78-ACSL4 Axis in Focus
- Intermittent Fasting: Metabolic and Gut Microbiota Insights for Fatty Liver Management
- How the TM6SF2-E167K Variant Drives Liver Disease: A Lipidomics Study
- Multi-Omics Association Analysis Series
- Omics Data Processing Series
- Omics Data Analysis Series
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


