Ion Formation and Organic Fragmentation in LCMS: Unlocking the Secrets of Mass Spectra
In liquid chromatography-mass spectrometry (LCMS) analysis, mass spectra provide a wealth of ion information that is crucial for the identification and structural elucidation of compounds. Understanding the fragmentation patterns of organic compounds and the mechanisms of ion formation is of paramount importance in mass spectrometry. These insights not only facilitate the rapid identification of target substances but also enable researchers to interpret complex spectra with greater accuracy and confidence. This detailed understanding of ion formation and fragmentation patterns is essential for advancing research in fields such as metabolomics, proteomics, and environmental analysis, where precise compound identification is critical. By mastering these concepts, researchers can unlock the full potential of LCMS technology, leading to more accurate and insightful analytical results.
Ion Formation Mechanism and Process in ESI Ion Source
The ion formation mechanism in LCMS is key to identifying target substances. Taking the electrospray ionization (ESI) source as an example, the ion formation process is as follows: Under the influence of a high-voltage electric field and auxiliary gas, charged droplets are sprayed out. As the solvent evaporates, the droplet volume gradually decreases, and the charge density increases continuously. When the Rayleigh limit is reached, the droplets undergo Coulomb explosion. Finally, ions evaporate from the droplets to form gaseous ions.
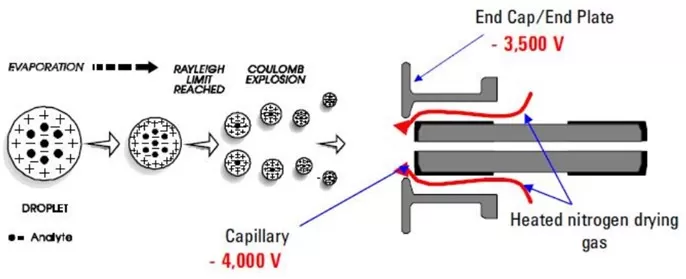
Ion formation process in ESI source
Top 6 Ion Sources in Mass Spectrometry: EI, CI, ESI, APCI, APPI, and MALDI
Common Ions in Mass Spectra
Since LCMS employs soft ionization techniques, the generated ions are typically "protonated/deprotonated or other adduct ions," referred to as quasi-molecular ions. Below are common ion types observed in full-scan mode mass spectra:
Common Ion Types in Positive Ion Mode:
- Protonated Molecular Ion [M+H]⁺: This is the most common ion type in positive ion mode, with a mass-to-charge ratio (m/z) increased by 1. It typically appears in nitrogen-containing compounds or others prone to protonation.
- Metal Adduct Ions [M+Na]⁺, [M+K]⁺: Sodium adduct ion [M+Na]⁺ (m/z +23) is common in sugars and alcohols. Potassium adduct ion [M+K]⁺ (m/z +39) usually exhibits lower signal intensity compared to sodium adducts.
- Ammonium Adduct Ion [M+NH₄]⁺: Formed when ammonium salts are present in the mobile phase (m/z +18), commonly observed in neutral or weakly polar compounds.
- Polymer Ions: At high concentrations, polymer ions such as protonated dimers [2M+H]⁺ or sodium adduct dimers [2M+Na]⁺ may form.
Common Ion Types in Negative Ion Mode:
- Deprotonated Molecular Ion [M-H]⁻: The most common ion in negative ion mode (m/z -1), typically observed in carboxylic acids, phenols, and other compounds prone to deprotonation.
- Halogen Adduct Ions: Chloride adducts [M+Cl]⁻ are observed in some neutral compounds, exhibiting characteristic isotopic peaks of chlorine.
- Other Adduct Ions: Formate adduct ion [M+HCOO]⁻ (m/z +45) forms when formic acid is present in the mobile phase. Acetate adduct ion [M+CH₃COO]⁻ (m/z +59) forms with acetic acid in the mobile phase.
Other Ions in Mass Spectra
Additionally, the following special ions may appear due to differences in compound properties:
- Fragment Ions: Generated from precursor ion cleavage, sharing the same elution time as the precursor.
- Isotopic Ions: Resulting from naturally occurring isotopes, with specific abundance ratios determined by the number and valency of isotopes.
- Multiply Charged Ions: Examples include [M+2H]²⁺ and [M+3H]³⁺, commonly observed in the analysis of large molecules such as proteins and peptides.
Fragmentation Patterns of Organic Compounds in Mass Spectrometry
The fragmentation patterns of organic compounds reveal how molecules break apart under specific conditions, providing valuable clues about their structural features. By analyzing these patterns, scientists can deduce the molecular structure, identify functional groups, and even predict the behavior of unknown compounds. Below are common fragmentation rules for different types of organic compounds:
1. Alkanes
C-H bonds are generally more stable than C-C bonds; thus, cleavage occurs between C-C bonds, following the "loss of the largest alkyl group" principle. Molecular ion peaks weaken significantly with increasing carbon chain length in linear alkanes, while branched alkanes may lack observable molecular ion peaks. Cleavage in branched alkanes occurs at substituted carbons due to the stability of resulting carbocations. Fragments follow the even-electron rule, generating 29+14n series ions (e.g., m/z 43, 57, 71, 85).
2. Alkenes
Cleavage occurs at allylic positions, forming resonance-stabilized carbocations (e.g., m/z 41). Fragments follow the 41+14n series. Compounds with double bonds may undergo McLafferty rearrangement, producing CnH2n ions.
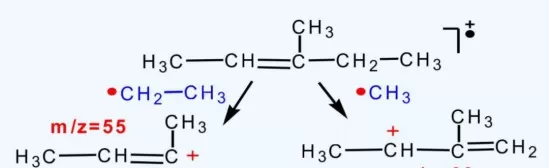
Fragmentation pattern of alkenes
3. Aromatic Hydrocarbons
Strong molecular ion peaks are observed, often with characteristic peaks at m/z 77 and 51. Benzyl cleavage generates benzyl ions. Alkyl-substituted aromatics undergo β-cleavage (due to double bonds in the benzene ring), forming troponium ions with a base peak at m/z 91.
4. Alcohols
α-cleavage occurs at the C-OH bond, producing resonance-stabilized hydroxy carbocations. Characteristic ions include: primary alcohols (m/z 31, CH₂=OH⁺), secondary alcohols (m/z 45, CH₃-CH=OH⁺), and tertiary alcohols (m/z 59, (CH₃)₂-C=OH⁺).
5. Aldehydes and Ketones
Molecular ion peaks are usually prominent. Aliphatic aldehydes exhibit M-1 peaks of comparable intensity to molecular ions. Primary cleavage is α-cleavage, yielding resonance-stabilized acylium ions and alkyl radicals. Long-chain aldehydes lose the aldehyde group, forming [M-29]+ peaks. McLafferty rearrangement in aldehydes produces m/z 44, while ketones yield m/z 58.
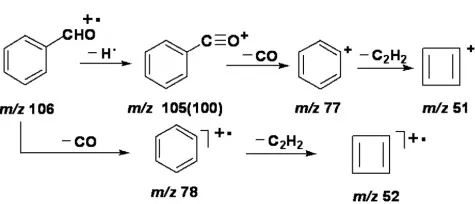
Fragmentation pattern of aldehydes and ketones
6. Esters
Small esters exhibit strong molecular ion peaks. Methyl esters may show M-31 (M-OCH₃) fragments, and ethyl esters M-45 (M-OC₂H₅). γ-H rearrangement generates m/z 74. Long-chain esters display dihydrogen rearrangement peaks.
7. Carboxylic Acids
Fatty acids show prominent molecular ion peaks. Cleavage occurs via α-cleavage and McLafferty rearrangement, yielding fragments such as M-17 (M-OH), M-45 (M-COOH), and m/z 45 (COOH). γ-H rearrangement produces m/z 60 and a series of 60+14n fragments.
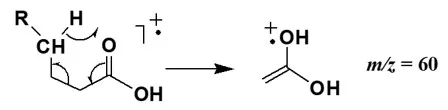
Fragmentation pattern of carboxylic acids
8. Amines
Molecular ion peaks of aliphatic amines are weak. Amines with odd nitrogen atoms exhibit odd molecular masses. Fragmentation resembles alcohols and ethers, favoring α-cleavage. Characteristic ions include primary amines (m/z 30), secondary amines (m/z 44), and tertiary amines (m/z 58).


