From Stability to Function: The Importance of Disulfide Bonds in Proteins
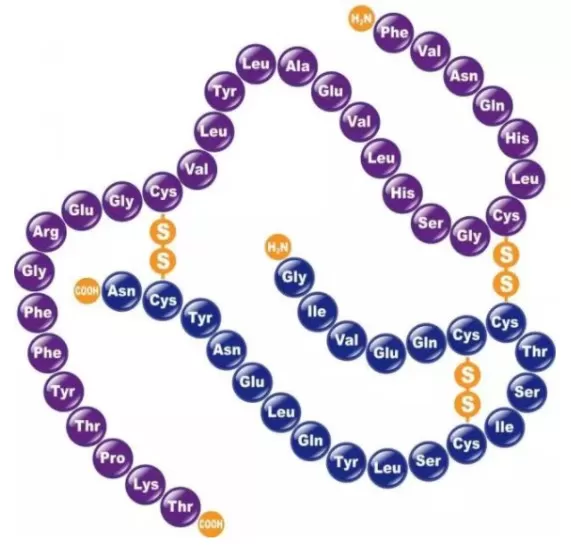 Disulfide bonds (S-S) in proteins are crucial for the formation and stabilization of their three-dimensional structures. These bonds are formed through covalent linkages between two cysteine residues and play a significant role in maintaining and regulating the stability, proper folding conformation, and biological activity of proteins. A comprehensive understanding of the formation, function, and analytical methods of disulfide bonds can enhance our grasp of complex biochemical processes.
Disulfide bonds (S-S) in proteins are crucial for the formation and stabilization of their three-dimensional structures. These bonds are formed through covalent linkages between two cysteine residues and play a significant role in maintaining and regulating the stability, proper folding conformation, and biological activity of proteins. A comprehensive understanding of the formation, function, and analytical methods of disulfide bonds can enhance our grasp of complex biochemical processes.
Formation of Disulfide Bonds
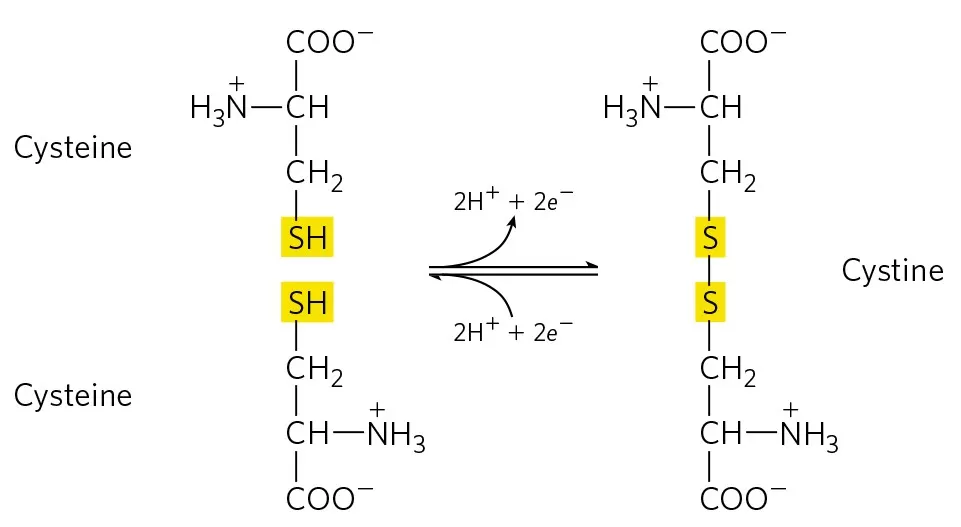
The formation of disulfide bonds is a radical reaction process. The thiol group of cysteine residues is first oxidized to form sulfur radicals, which then combine to create disulfide bonds through the sharing of electron pairs. This reaction is irreversible and is tightly regulated within biological systems.
1. Oxidation Stage: In the endoplasmic reticulum, the sulfur atom of cysteine residues is oxidized by agents such as Ero1, resulting in highly reactive sulfur ions.
2. Exchange Stage: The oxidized cysteine residues can form covalent bonds with sulfur ions from another protein chain, typically occurring during the protein folding process. This process can be intramolecular, where disulfide bonds are formed within the same polypeptide chain, or intermolecular, where bonds form between two different polypeptide chains. This process is regulated by a series of enzymes, including protein disulfide isomerase (PDI).
Biological Significance of Disulfide Bonds
Disulfide bonds, formed between the sulfur atoms of two cysteine residues, play a critical role in the structure and function of proteins. Here are some key aspects of their biological significance:
Structural Stability:
Disulfide bonds contribute significantly to the stabilization of protein tertiary and quaternary structures, especially in extracellular and secreted proteins. By creating covalent linkages, they help maintain the correct folding of proteins under varying environmental conditions, such as changes in temperature and pH.
Oxidative Folding:
In the endoplasmic reticulum (ER), where proteins are synthesized and folded, disulfide bond formation is essential for oxidative folding. This process ensures that proteins acquire their functional conformations, critical for their biological activity.
Functional Roles:
Disulfide bonds can influence the functional properties of proteins. For instance, in enzymes, they may play a role in substrate binding or catalytic activity. In receptors, disulfide bonds can affect ligand binding and signal transduction.
Cellular Localization:
Proteins that contain disulfide bonds are often secreted or localized to the extracellular matrix, where they perform important functions, such as structural support and cell signaling. The formation of disulfide bonds helps distinguish these proteins from those that remain within the cytoplasm.
Protection Against Oxidative Stress:
Disulfide bonds can serve as redox-sensitive switches, modulating protein activity in response to changes in the cellular oxidative environment. This ability allows cells to adapt to oxidative stress, maintaining homeostasis.
Functions of Disulfide Bonds in Human Diseases
Disulfide bonds play a crucial role in maintaining protein structure and function, and their dysregulation can lead to various human diseases.
Neurodegenerative Diseases:
Disulfide bonds help stabilize protein structures, and incorrect formation can lead to misfolded proteins that aggregate in cells. For instance, in Alzheimer’s disease, amyloid-beta peptides form disulfide-linked oligomers that disrupt cellular function and promote neuroinflammation. Similarly, in Parkinson’s disease, misfolded alpha-synuclein proteins form toxic aggregates that impair neuronal health.
Cystic Fibrosis:
In cystic fibrosis, mutations in the CFTR protein often lead to improper disulfide bond formation. This misfolding prevents CFTR from reaching the cell surface, where it is essential for chloride ion transport. The resulting ion imbalance contributes to thick mucus production in the lungs, making patients susceptible to infections and respiratory complications.
Cancer and Tumor Progression:
Cancer cells often exhibit altered disulfide bond dynamics, which can affect signaling pathways involved in cell proliferation and survival. For example, the enzyme thioredoxin can reduce disulfide bonds in proteins, altering their activity. This redox regulation can enhance the proliferation of cancer cells and contribute to their resistance to chemotherapy by protecting them from oxidative stress.
Autoimmune Diseases:
In autoimmune diseases like systemic lupus erythematosus (SLE), oxidative stress can lead to the formation of aberrant disulfide bonds in self-antigens. This can create new epitopes recognized by the immune system as foreign, triggering an autoimmune response. Elevated levels of oxidized proteins can be biomarkers for disease activity.
Diabetes:
In diabetes, hyperglycemia can lead to increased oxidative stress, affecting disulfide bond formation in proteins like insulin. This can result in insulin misfolding or reduced activity, disrupting glucose homeostasis. Furthermore, altered disulfide bonds in insulin receptors can impair their signaling efficiency, contributing to insulin resistance.
Cardiovascular Diseases:
Disulfide bonds in proteins regulating vascular tone and endothelial function can be disrupted by oxidative stress. For example, alterations in disulfide bonds in endothelial nitric oxide synthase (eNOS) can impair nitric oxide production, leading to endothelial dysfunction and promoting atherosclerosis. Dysregulated disulfide bonds in matrix proteins can also impact vascular remodeling and stiffness.
Infectious Diseases:
Certain pathogens produce proteins that target host cell disulfide bonds to enhance virulence. For example, the bacterial protease Staphyloxanthin can cleave disulfide bonds in host proteins, facilitating bacterial invasion. Additionally, some viruses can induce oxidative stress in host cells, leading to the improper formation of disulfide bonds that aid in viral replication and pathogenesis.
Age-Related Diseases:
Aging is associated with changes in the cellular redox environment, leading to increased formation of improper disulfide bonds in proteins. This contributes to the accumulation of damaged proteins, which is a hallmark of age-related diseases like Alzheimer’s and cardiovascular diseases. The resulting loss of protein function exacerbates cellular stress and contributes to the decline in tissue homeostasis.
Analytical Methods for Disulfide Bonds
Protein identification and structural analysis are essential components of biological research. We can predict disulfide bonds in proteins using online tools such as the Prosite database. For further confirmation, various analytical techniques can be employed, including mass spectrometry, X-ray crystallography, and nuclear magnetic resonance (NMR) spectroscopy.
1. LC-MS (Liquid Chromatography-Mass Spectrometry): LC-MS combines liquid chromatography with mass spectrometry, providing a powerful approach for analyzing disulfide bonds in proteins. In this method, proteins are first digested into peptides, and liquid chromatography is used to separate these peptides based on their hydrophobicity. The separated peptides are then analyzed by mass spectrometry, which measures their mass-to-charge ratios. This dual technique allows for the identification of disulfide-containing peptides and provides fragmentation patterns that indicate the presence and positioning of disulfide bonds, making it essential for detailed structural analysis of complex protein modifications.
2. Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy offers atomic-level insights into protein structures, including the characteristics of disulfide bonds. This technique measures the magnetic properties of atomic nuclei in proteins, revealing information about the environment of sulfur atoms in disulfide linkages. While NMR is advantageous for studying protein dynamics in solution, it typically requires high concentrations of purified proteins and may be challenging for larger complexes.
3. X-ray Crystallography: X-ray crystallography enables visualization of protein structures at atomic resolution, allowing for the precise determination of disulfide bond locations. By crystallizing proteins and directing X-rays at the crystals, diffraction patterns are generated and analyzed to reveal the three-dimensional arrangement of atoms. This method is invaluable for detailed structural studies, particularly for elucidating disulfide bond positioning in the context of overall protein architecture.
4. Enzymatic Assays: Enzymatic assays leverage enzymes like protein disulfide isomerase (PDI) to study disulfide bond formation and isomerization. By observing changes in protein activity or conformation in the presence of PDI, researchers can infer the dynamics and stability of disulfide bonds during protein folding. This method provides functional insights into the roles disulfide bonds play in maintaining protein structure.
5. Circular Dichroism (CD) Spectroscopy: Circular dichroism spectroscopy assesses the optical activity of proteins, offering insights into their secondary structure that may be influenced by disulfide bonding. By measuring the differential absorption of left- and right-handed circularly polarized light, CD can detect conformational changes in proteins, especially those resulting from disulfide bond formation or reduction, making it a useful tool for studying protein folding.
6. Chemical Methods: Chemical methods for analyzing disulfide bonds often involve reduction and alkylation processes. Reducing agents such as DTT or TCEP are used to cleave disulfide bonds, followed by alkylation with agents like iodoacetamide to stabilize the resulting thiols for further analysis. Specific labeling of thiol groups can also facilitate the identification of disulfide bond locations after reduction, enhancing understanding of protein structure.
7. Fluorescence Spectroscopy: Fluorescence spectroscopy utilizes fluorescent probes that selectively bind to thiol groups, enabling real-time detection of disulfide bond dynamics. This method allows researchers to monitor conformational changes in proteins during folding or under varying conditions, providing insights into the role of disulfide bonds in maintaining protein functionality within living cells.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


