Exploring Brain Lipids: Composition, Synthesis, Functions, Disease Connections, and Detection Techniques
When we think about brain health, we often focus on neurons, neurotransmitters, and synapses. But did you know that lipids, the fats that form the structural foundation of our brain cells, are equally crucial for cognitive function and mental well-being? These fascinating molecules are more than just building blocks—they play vital roles in everything from cell signaling to protecting against neurodegenerative diseases. In this blog, we’ll delve into the intricate world of brain lipids, exploring their various types, functions, and the cutting-edge detection methods that researchers use to study them. Whether you're curious about how brain lipids contribute to cognitive health or their links to disorders like Alzheimer's and Parkinson's, this guide will provide a comprehensive overview of these unsung heroes of the brain. Let’s unlock the secrets of brain lipids and discover how they shape our mental health and beyond.
- Diverse Lipid Landscape: Understanding Brain Lipid Composition
- Lipid Synthesis Unveiled: Pathways and Processes in the Brain
- How Lipids Power the Brain: Key Functions and Roles
- Brain Lipids and Neurological Diseases: Alzheimer's, Parkinson's, and Beyond
- Cutting-Edge Lipid Analysis: Advanced Detection Methods at MetwareBio
Diverse Lipid Landscape: Understanding Brain Lipid Composition
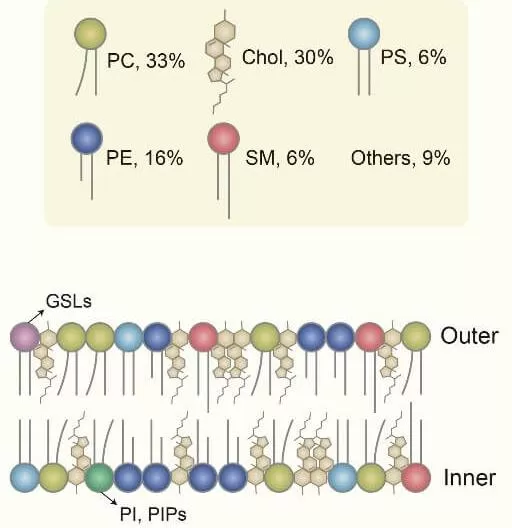 The brain is often thought of as a hub of electrical activity, but beneath this energetic surface lies a complex and diverse landscape of lipids. Lipids are fundamental components of cell membranes and are primarily synthesized by enzymes in the endoplasmic reticulum. They form the very fabric of our brain cells, creating a dynamic environment that supports everything from membrane structure and fluidity to cellular communication. Among all human organs and tissues, the brain has the highest lipid content after adipose tissue, accounting for 50% of its dry weight. The brain’s lipid composition primarily consists of cholesterol (30%), glycerophospholipids (55%), including phosphatidylcholine (33%), phosphatidylethanolamine (16%), and phosphatidylserine (6%), as well as sphingolipids (6%) and other lipid types (9%).
The brain is often thought of as a hub of electrical activity, but beneath this energetic surface lies a complex and diverse landscape of lipids. Lipids are fundamental components of cell membranes and are primarily synthesized by enzymes in the endoplasmic reticulum. They form the very fabric of our brain cells, creating a dynamic environment that supports everything from membrane structure and fluidity to cellular communication. Among all human organs and tissues, the brain has the highest lipid content after adipose tissue, accounting for 50% of its dry weight. The brain’s lipid composition primarily consists of cholesterol (30%), glycerophospholipids (55%), including phosphatidylcholine (33%), phosphatidylethanolamine (16%), and phosphatidylserine (6%), as well as sphingolipids (6%) and other lipid types (9%).
Lipid Synthesis Unveiled: Pathways and Processes in the Brain
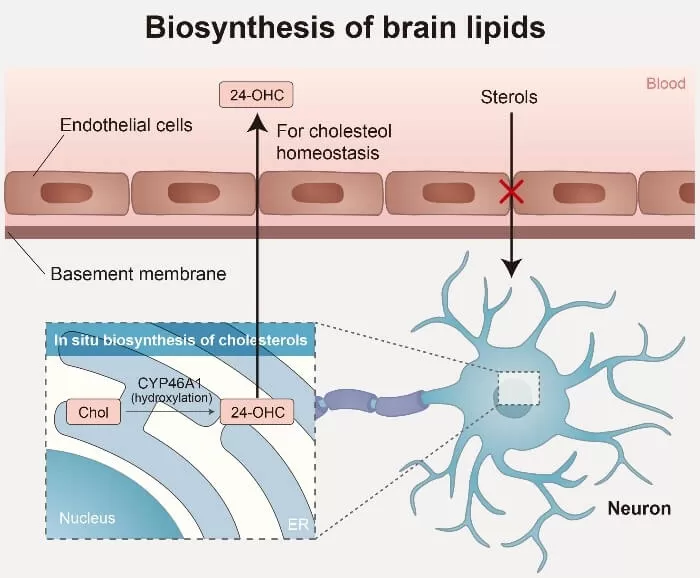 The brain has a notably high cholesterol content compared to other organs, with most of it synthesized locally due to the blood-brain barrier (BBB) restricting blood sterol entry. However, free cholesterol, particularly 24-hydroxycholesterol, can cross the BBB, playing a crucial role in maintaining cholesterol balance within the central nervous system. The synthesis of phospholipids and sphingolipids is initiated by fatty acids (FAs), which are essential components of most types of lipids. The brain mostly produces saturated FAs, while its ability to synthesize polyunsaturated FAs (PUFAs) is relatively poor. Due to limited local synthesis of polyunsaturated fatty acids (PUFAs), these essential lipids are mostly obtained from the bloodstream through passive diffusion or specific transport mechanisms.
The brain has a notably high cholesterol content compared to other organs, with most of it synthesized locally due to the blood-brain barrier (BBB) restricting blood sterol entry. However, free cholesterol, particularly 24-hydroxycholesterol, can cross the BBB, playing a crucial role in maintaining cholesterol balance within the central nervous system. The synthesis of phospholipids and sphingolipids is initiated by fatty acids (FAs), which are essential components of most types of lipids. The brain mostly produces saturated FAs, while its ability to synthesize polyunsaturated FAs (PUFAs) is relatively poor. Due to limited local synthesis of polyunsaturated fatty acids (PUFAs), these essential lipids are mostly obtained from the bloodstream through passive diffusion or specific transport mechanisms.
How Lipids Power the Brain: Key Functions and Roles
Lipids are vital for brain function, supporting everything from cell membrane structure to neurotransmission. Beyond being building blocks, they also aid in energy storage, synaptic plasticity, and neuron protection.
Cholesterol
The brain contains about 25% of the body’s total cholesterol. Most of this cholesterol is produced by astrocytes and is transferred to neurons via cholesterol-rich lipoproteins, such as apolipoprotein E (APOE), where it supports the maintenance of axons and synaptic connections. In the brain, endogenous cholesterol is primarily found in the myelin sheaths, and the levels of unesterified cholesterol are significantly higher compared to other tissues. Only around 1% of brain cholesterol is esterified and stored within cells as lipid droplets, serving as a reservoir for excess cholesterol. The BBB largely restricts the exchange of sterols between the CNS and peripheral tissues, with less than 1% of cholesterol being exchanged daily. Consequently, brain cholesterol metabolism operates almost independently of the rest of the body. Maintaining proper cholesterol homeostasis is crucial for normal brain function, and disruptions can lead to neurodegenerative diseases and cognitive impairment in the elderly.
Glycerophospholipids
Glycerophospholipids, including phosphatidylcholine (PC) and phosphatidylethanolamine (PE), are the primary phospholipid components of cell membranes. Alterations in their composition can impact the stability, permeability, and fluidity of neuronal membranes, potentially leading to neurological disorders. In brain cells, as in other tissues, PC and PE play crucial roles in anchoring proteins to membranes. Additionally, phospholipases A, C, and D catalyze the receptor-mediated degradation of glycerophospholipids, generating second messengers such as diacylglycerol (DAG), inositol 1,4,5-trisphosphate, lysophospholipids, platelet-activating factor, and long-chain polyunsaturated fatty acids (LCPUFAs). Notably, LCPUFAs are precursors to eicosanoids and docosanoids, which have significant neuroprotective and anti-inflammatory roles in the central nervous system.
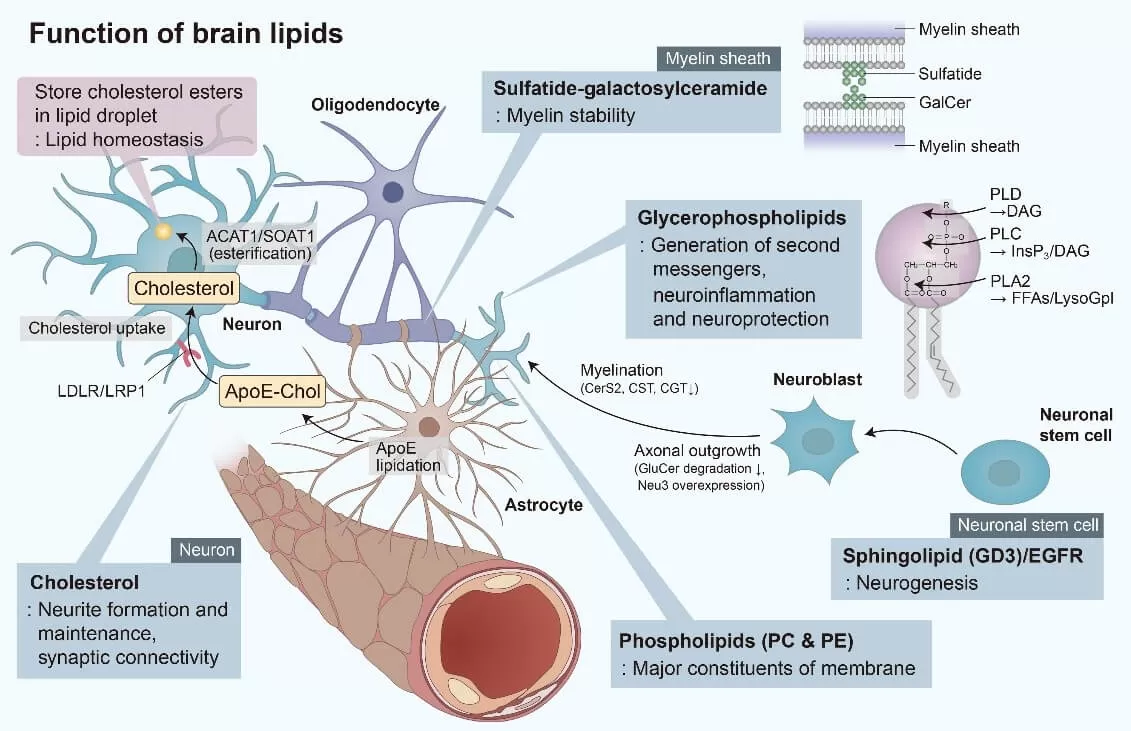
Sphingolipids
Sphingolipids, commonly found in the nervous system, are critical components of cellular membranes, playing essential roles in brain function, particularly in neurogenesis and synaptogenesis. Within lipid rafts, sphingolipids, together with cholesterol, regulate the activity of transmembrane proteins. In synaptic membranes, sphingolipids interact with neurotransmitter receptors, modulating their function.
Key sphingolipids include sphingomyelin (SM), gangliosides, cerebrosides, and sulfatides—all derived from ceramide (N-acetylsphingosine). SM, a major constituent of myelin, is abundant in white matter, as are cerebrosides, which are found in higher concentrations in white matter than in gray matter. Most cerebrosides in the brain are galactosylceramides, which, along with sulfatides, contribute to the long-term stability of myelin. Gangliosides, a type of glycosphingolipid containing sialic acid, are abundant in the central nervous system and are involved in cell signaling and neuroprotection. During neuronal stem cell proliferation, the ganglioside GD3 initially colocalizes with the epidermal growth factor receptor (EGFR) in cell microdomains. This process subsequently promotes neurite outgrowth in neuroblasts by inhibiting the degradation of glycosylceramides and overexpressing N-acetylneuraminidase 3 (Neu3).
Brain Lipids and Neurological Diseases: Alzheimer's, Parkinson's, and Beyond
As the global population ages, brain disorders such as neurodegenerative diseases, mental illnesses, and brain tumors are increasingly recognized as leading causes of morbidity and mortality. Notably, most neurodegenerative diseases are still diagnosed based on clinical symptoms—such as cognitive decline, motor dysfunction, and communication difficulties—while their underlying pathological mechanisms remain unclear, limiting the availability of effective treatments. Lipids are vital to cellular function, playing key roles in membrane formation, cell signaling, energy storage, and the maintenance of homeostasis. In the brain, lipid imbalances have been implicated in the onset and progression of neurodegenerative and other neurological disorders. As a result, brain lipids are emerging as important potential targets for the early diagnosis and prognosis of neurological diseases.
Alzheimer’s Disease
Alzheimer's disease (AD) is the most prevalent neurodegenerative disorder and the leading cause of dementia. It is widely recognized that AD is characterized by the presence of amyloid plaques and neurofibrillary tangles in the brain. Brain lipid metabolism plays a crucial role in the processing of amyloid precursor protein (APP), the production of β-amyloid (Aβ), and the aggregation of Aβ in Alzheimer’s disease (AD). Studies have shown that in AD, the transport of cholesterol from astrocytes to neurons is disrupted, which may be closely linked to apolipoprotein E (APOE). APOE, the primary cholesterol carrier in the brain, can bind and clear Aβ. Previous research indicates that a variant of the APOE gene is a risk factor for AD and is associated with alterations in cholesterol and sphingolipid levels. Lipid rafts, which are membrane microdomains rich in cholesterol and sphingolipids, anchor AD-related transmembrane proteins such as β-site APP-cleaving enzyme 1 (BACE1) and γ-secretase. The activity of these enzymes is influenced by cholesterol levels, suggesting that cholesterol metabolism plays a significant role in AD pathogenesis.
Sphingolipid metabolism is also highly related to the formation of Aβ oligomers, particularly Aβ42. Accumulation of sphingomyelin decreases γ-secretase activity, thereby reducing Aβ secretion. In AD patients, levels of acidic sphingomyelinase and acidic ceramidase are significantly elevated, leading to decreased sphingomyelin and increased ceramide production. Ceramide stabilizes BACE1 and extends its half-life, thus enhancing the rate of Aβ formation. Additionally, glycosphingolipids contribute to amyloid fibril formation. Overall, sphingolipids influence key processes in AD, including APP processing, Aβ production, and subsequent amyloid aggregation.
Phospholipids are linked to the activity of γ-secretase and other proteins involved in the processing of APP. Studies on the brains of AD patients have reported alterations in various phospholipid types, including phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylinositol (PI), as well as changes in the enzymes that regulate their metabolism, such as phospholipase C (PLC) and phospholipase D (PLD). In the context of AD-related cognitive impairment, the loss of phospholipid transfer proteins has been shown to reduce PE and PI levels, which accelerates intracellular Aβ accumulation and leads to memory deficits. These findings suggest a connection between phospholipid metabolism and APP processing, highlighting the potential role of phospholipids in AD pathogenesis.
 by lipids_1727160223_WNo_718d516.webp)
Parkinson’s Disease
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, primarily affecting individuals over the age of 60. PD symptoms include motor impairments such as tremors, bradykinesia, rigidity, and balance issues. In addition to these motor symptoms, cognitive changes are also common in PD patients and can often progress to dementia. Over the past few decades, research has implicated several cellular pathways in PD, including oxidative stress, lysosomal dysfunction, endoplasmic reticulum stress, and immune responses.
A hallmark of PD is the accumulation of α-synuclein, a neuronal protein that regulates synaptic vesicles, neurotransmitter release, and contributes to the formation of Lewy bodies, as well as the loss of dopaminergic neurons. Under normal physiological conditions, α-synuclein helps maintain synaptic stability and regulates dopamine synthesis. However, in PD patients, abnormal aggregation of α-synuclein occurs, forming Lewy bodies (fibrous aggregates of α-synuclein) and leading to the degeneration of dopaminergic neurons.
Additionally, in PD patients, levels of triglycerides are reduced and diacylglycerol levels are elevated in the primary visual cortex. Phospholipase D1, which inhibits α-synuclein accumulation through autophagic flux, has reduced activity in PD patients, exacerbating α-synuclein toxicity. Changes in lipid composition within lipid rafts have also been observed in the frontal cortex of human PD donors, further highlighting the role of lipid metabolism in the disease.
Huntington's Disease
Huntington's disease (HD), also known as Huntington's chorea, is an autosomal dominant neurodegenerative disorder characterized by abnormal motor functions (chorea and dystonia), psychiatric complications (anxiety and depression), and cognitive impairments (dementia). The mutation of the huntingtin (HTT) gene is a well-established cause of HD. The HTT gene and its related proteins are involved in various intracellular functions, including postsynaptic signaling, protein transport, and protein aggregation.
In patients with Huntington's disease, levels of sphingosine-1-phosphate lyase 1 (a key enzyme in sphingolipid metabolism) are elevated in the striatum and cortex, while sphingosine kinase 1 levels are reduced, indicating a disturbance in sphingolipid metabolism. Additionally, studies of human samples have revealed disruptions in neurocholesterol and cholesterol ester (CE) levels in HD. Notably, the caudate nucleus and putamen of HD patients show increased CE levels, which may subsequently reduce cholesterol accumulation.
Schizophrenia
Schizophrenia (SCZ) is a mental disorder characterized by disturbances in thought and behavior, along with symptoms such as hallucinations and delusions. It is often inadequately treated with antipsychotic medications. The neurobiology of SCZ can be explained by alterations in dopaminergic, glutamatergic, and serotonergic signaling pathways.
Research has shown that membrane phospholipid composition in the cortical tissue of SCZ patients is abnormal, with changes in lipid composition potentially affecting neurotransmitter storage and release. Compared to healthy individuals, there are significant changes in the concentration of lipids in the prefrontal cortex (PFC) of SCZ patients, with an average alteration of 10.4%, which may enhance membrane fluidity. Assessments of biomarkers in serum samples from SCZ patients indicate lower levels of phosphatidylethanolamine (PE), phosphatidylcholine (PC), and lysophosphatidylcholine (LPC), while levels of sphingomyelins (SMs) and most lysophosphatidylethanolamines (LPEs) are elevated compared to controls.
Cutting-Edge Lipid Analysis: Advanced Detection Methods at MetwareBio
A variety of techniques are employed in brain lipidomic studies, including nuclear magnetic resonance (NMR) spectrometry, fluorescence assays, and mass spectrometry (MS). Each method offers distinct analytical performances in terms of sensitivity and efficiency, and the choice of technique largely depends on the specific objectives of the research. Fluorescence assays are among the simplest methods for quantifying specific lipid components; however, they are not well-suited for comprehensive lipid profiling. In contrast, while NMR is less sensitive than MS-based approaches, it provides advantages such as non-destructive sample preparation and the capability for detailed structural elucidation. Recent advancements in instrumental performance have led to the adoption of techniques such as shotgun MS, liquid chromatography–mass spectrometry (LC-MS), and gas chromatography–mass spectrometry (GC-MS) in lipidomic research. State-of-the-art MS technologies exhibit both high sensitivity and high resolution, facilitating in-depth analysis of the composition and structure of a wide array of lipid species.
At MetwareBio, we are leading the way in lipidomics with our cutting-edge quantitative lipid analysis services tailored for researchers focused on brain and neuro-related diseases. Our MS-based detection methods harness the power of high-resolution mass spectrometry to provide precise and comprehensive lipid profiles, crucial for understanding lipid metabolism in the context of neurological health. Whether you're investigating the roles of lipids in neurodegenerative diseases or exploring their impact on brain function, our extensive databases and stringent quality control protocols ensure reliable and reproducible results.
|
Number of Lipids |
||
|
Class I |
Class II |
Number |
|
Fatty acyls(FA) |
CAR, FFA, Eicosanoid, FAHFA |
270 |
|
Glycerolipids(GL) |
DG, DG-O, MG, TG, TG-O, MGDG, DGDG |
1015 |
|
Glycerophospholipids(GP) |
LPC, LPC-O, LPE, LPE-P, LPG, LPS, PC, PC-O, PE, PE-P, PE-O, PG, PS, LPI, PI, LPA, PA, PMeOH, BMP, HMBP, LNAPE |
1800 |
|
Sphingolipids(SL) |
SPH, CerP, HexCer, SM, Cer, Cert |
828 |
|
Sterol lipids(ST) |
Cho, CE, BA, CASE |
122 |
|
Prenol lipids(PR) |
CoQ |
3 |
|
Total |
4000+ |
|
In addition, MetwareBio offers comprehensive multi-omics joint analysis services to our clients, enabling a holistic approach to understanding complex biological systems. By integrating lipidomics with other omics disciplines, such as genomics, transcriptomics, proteomics, and metabolomics alongside clinical data, we can uncover intricate molecular networks associated with various pathological phenotypes. This integrated analysis allows for the identification of biomarkers and potential therapeutic targets that may not be evident when examining individual omics layers in isolation. By leveraging the synergy between different types of data, we can provide deeper insights into the underlying mechanisms of diseases, facilitating more informed decision-making in research and clinical applications. Our advanced analytical platforms ensure that the integration process is seamless and efficient, delivering robust results that enhance the understanding of disease mechanisms and inform personalized medicine strategies.
Partner with us to unlock new insights into lipid signaling pathways and their implications in neurobiology, empowering your research with the highest standards in lipidomics and multi-omics analyses.
Reference:
Yoon, J. H., Seo, Y., Jo, Y. S., Lee, S., Cho, E., Cazenave-Gassiot, A., Shin, Y. S., Moon, M. H., An, H. J., Wenk, M. R., & Suh, P. G. (2022). Brain lipidomics: From functional landscape to clinical significance. Science advances, 8(37), eadc9317. https://doi.org/10.1126/sciadv.adc9317
Di Paolo, G., & Kim, T. W. (2011). Linking lipids to Alzheimer's disease: cholesterol and beyond. Nature reviews. Neuroscience, 12(5), 284–296. https://doi.org/10.1038/nrn3012
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


