Exciting Upgrade to Our Energy Metabolism Targeted Metabolomics Service
Energy metabolism plays a central role in cellular function, providing the necessary energy for biological processes such as cell growth, differentiation, and homeostasis. Understanding energy metabolism is not only fundamental for basic biological research but also essential for uncovering the underlying mechanisms of various diseases, including cancer, diabetes, and neurodegenerative disorders. Three key pathways—glycolysis, the tricarboxylic acid (TCA) cycle, and the pentose phosphate pathway—are central to energy metabolism, orchestrating the transformation of nutrients into biochemical energy and essential biomolecules. To help researchers gain deeper insights into these intricate metabolic pathways, our energy metabolism targeted metabolomics service has recently undergone a comprehensive upgrade. This enhanced service offers expanded detection coverage, improved quantification accuracy, and optimized quality control—delivering more reliable and comprehensive data for your research.
Expanded Database: Broader Detection Coverage
In the upgraded version, the database of detectable metabolites has been significantly expanded. The original panel could detect 68 compounds across three key metabolic pathways: the TCA cycle, pentose phosphate pathway, and glycolysis. These pathways are interconnected, playing critical roles in cellular energy production, redox balance, and biosynthesis. The TCA cycle is the central hub of aerobic metabolism, generating ATP and electron carriers such as NADH and FADH2. Glycolysis provides the initial breakdown of glucose into pyruvate, supplying energy and intermediates for other metabolic pathways. Meanwhile, the pentose phosphate pathway produces NADPH for antioxidant defense and ribose-5-phosphate for nucleotide synthesis. With the upgrade, the panel now covers 80 compounds, adding 12 new metabolites to provide a more comprehensive analysis of cellular energy metabolism. This expanded detection capacity enables researchers to gain more detailed insights into the dynamic changes of energy metabolism under different biological conditions.
Information of 12 compounds newly added to the energy metabolism database
|
Metabolite |
Class |
Pathway |
|
L-2-Hydroxyglutaric acid |
Carbohydrate metabolism |
Upstream of the TCA Cycle |
|
Glycolic acid |
Carbohydrate metabolism |
Upstream of the TCA Cycle |
|
2-Oxoadipic acid |
Carbohydrate metabolism |
Downstream of the TCA Cycle/Amino Acid Metabolism |
|
DL-Glyceric Acid |
Carbohydrate metabolism |
Pentose phosphate pathway |
|
D-Ribose 5-phosphate |
Phosphate sugars |
Pentose phosphate pathway |
|
Gluconate |
Carbohydrate metabolism |
Pentose Phosphate Pathway |
|
D-ribulose-1,5-bisphosphate |
Phosphate sugars |
Pentose Phosphate Pathway |
|
Triphosphate guanosine |
Nucleotide and Its metabolites |
Downstream of the Pentose Phosphate Pathway/Purine Metabolism |
|
Ureidopropionate |
Carbohydrate metabolism |
Downstream of the Pentose Phosphate Pathway/Pyrimidine Metabolism |
|
Glucuronic acid |
Carbohydrate metabolism |
Upstream of the Pentose Phosphate Pathway/Glucuronic Acid Pathway |
|
D-Mannose-6-phosphate |
Phosphate sugars |
Upstream of Glycolysis/Fructose and Mannose Metabolism |
|
Cysteic acid |
Carbohydrate metabolism |
Downstream of Glycolysis |
Improved Quantification with Internal Standards
Accurate quantification is critical in metabolomics research, as small fluctuations in metabolite concentrations can have significant biological implications. The previous panel relied solely on external standards for quantification, which may be affected by sample matrix effects and experimental variability. The upgraded version introduces 32 internal standards, enabling 72 compounds to be quantified using both internal and external calibration curves. Internal standards are structurally similar to target metabolites and are spiked into samples at known concentrations, serving as references to correct for signal variations during sample processing and LC-MS/MS analysis. With internal standards incorporated into the calibration curve, the quantitation accuracy is significantly improved, offering more robust and consistent results across different samples and batches.
Enhanced Quality Control for Higher Data Reliability
To further ensure data accuracy, the upgraded panel includes an improved quality control strategy. A blank subtraction control measure has been introduced:
- If the metabolite concentration in the experimental sample is less than three times the concentration in the blank sample, the result is reported as NA (Not Detected).
- If the metabolite concentration exceeds three times the blank concentration, the final reported concentration is the measured value minus the average concentration from the blank samples.
This additional quality control step effectively eliminates background noise, enhancing the reliability of the quantitative results. Background signals from solvents, reagents, or instrument contamination can interfere with the detection of low-abundance metabolites, potentially leading to false positives. By applying the blank subtraction control, the upgraded panel minimizes such interference, ensuring that the reported metabolite concentrations truly reflect the biological sample.
Comprehensive Methodological Validation
The upgraded panel has undergone rigorous methodological validation using five different sample types, including mammalian cells, plant tissues, and biofluids. Approximately 90% of the compounds show recovery rates ranging from 60% to 140%, demonstrating good accuracy in diverse sample matrices. Additionally, nearly all compounds (around 100%) exhibit intra-day and inter-day precision with CV values below 30%, reflecting the method’s high reproducibility and reliability. Methodological validation is essential for establishing the robustness and consistency of metabolomics data, providing researchers with confidence in the accuracy of their results.
Optimized Plant Sample Preprocessing
In response to the unique challenges posed by plant samples, the upgraded panel features an optimized preprocessing method. Plant tissues are rich in secondary metabolites, pigments, and polysaccharides, which can interfere with metabolite extraction and LC-MS/MS analysis. The new protocol incorporates dichloromethane into the extraction solvent, which improves the liquid chromatography (LC) process by enhancing the separation of polar and non-polar compounds. This modification not only increases the extraction efficiency of energy metabolites but also reduces the risk of LC column clogging, which is a common issue when analyzing plant samples. The optimized preprocessing method enhances the overall efficiency and robustness of the analysis, particularly for complex plant matrices.
Proven Experience Across Diverse Sample Types
Our upgraded energy metabolism targeted metabolomics service has already been successfully applied to various biological sample types, including human and animal tissues, plant materials, and microorganisms. Across these diverse matrices, we have detected an average of 68+ metabolites, showcasing the panel’s robust performance and wide applicability. This experience highlights the upgraded panel’s capability to deliver consistent and reliable data across different biological systems, laying the groundwork for more comprehensive studies in energy metabolism. The expanded coverage and improved quantification accuracy further enhance its potential to support a broader range of research needs.
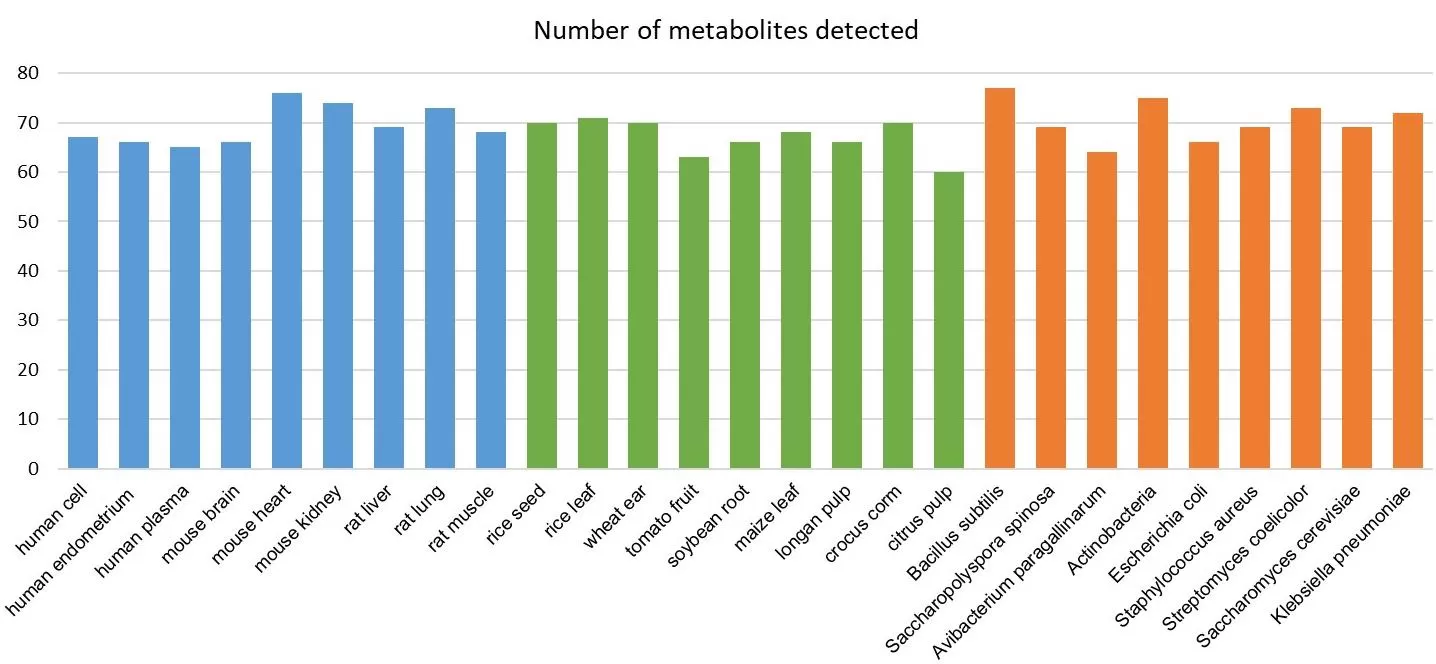
Past experience on various sample types of human/animals, plants and microorganisms
Drive Your Research with MetwareBio’s Energy Metabolism Detection
With expanded detection coverage, improved quantification accuracy, enhanced quality control, and optimized preprocessing methods, our upgraded energy metabolism targeted metabolomics service empowers researchers with more comprehensive and reliable data. These advancements will help researchers better understand the dynamic changes of energy metabolism in health, disease, and various biological systems. This upgrade reflects our ongoing commitment to supporting cutting-edge research in energy metabolism and beyond.
Unlock the full potential of your research with our advanced energy metabolism-targeted metabolomics service. With precise detection and quantification of key metabolites involved in energy metabolism, we can help you gain deeper insights into cellular processes, disease mechanisms, and therapeutic developments. Partner with us today to take your research to the next level. Contact us now to learn more or get started!
Read more
- Spatial Metabolomics: Transforming Biomedical and Agricultural Research
- Spatial Metabolomics Explained: How It Works and Its Role in Cancer Research
- MALDI, DESI, or SIMS? How to Choose the Best MSI Techniques for Spatial Metabolomics
- LC vs. HPLC vs. UHPLC: Tracing the Evolution of Chromatographic Techniques
- Mastering Chromatography: Everything You Need to Know
- DIA Proteomics vs DDA Proteomics: A Comprehensive Comparison
- Multi-Omics Association Analysis Series
- Omics Data Processing Series
- Omics Data Analysis Series


