ELISA vs. Western Blot: Choosing the Best Immunoassay for Your Research
In the field of life sciences, immunoassays serve as powerful tools for detecting and quantifying biological molecules such as proteins, antibodies, and antigens. These techniques are fundamental in areas ranging from basic biological research to clinical diagnostics and drug development. Among the most widely used immunoassay methods, ELISA (Enzyme-Linked Immunosorbent Assay) and Western Blot stand out due to their precision and reliability. However, despite their shared purpose of detecting target molecules, these two methods differ significantly in their principles, applications, advantages, and limitations. Understanding the strengths and weaknesses of each method is crucial for researchers when selecting the most suitable approach for their specific experimental needs. This blog provides an in-depth comparison between ELISA and Western Blot, offering detailed insights into their methodologies, best-use cases, and how they can complement each other in scientific research.
What is ELISA?
ELISA is a widely used immunoassay technique designed to detect and quantify specific proteins, antigens, or antibodies within a biological sample. The method is known for its high sensitivity, specificity, and throughput, making it an ideal choice for large-scale sample screening. ELISA can detect proteins at concentrations as low as 0.01 nanograms per milliliter (ng/mL), making it a preferred tool for precise quantification.
This assay is typically performed in 96-well microplates, where antigen-antibody interactions lead to an enzymatic reaction that generates a measurable signal. The intensity of this signal is directly proportional to the concentration of the target molecule, allowing for accurate quantification using a standard curve.
Step-by-Step Process of ELISA
The ELISA procedure involves several critical steps, each of which contributes to the specificity and reliability of the assay.
1. Coating (Immobilization of Target Molecule): The first step involves binding either the antigen or antibody to the surface of a solid support, typically a polystyrene microplate. This immobilization ensures that the target molecule is securely attached and available for interaction in subsequent steps.
2. Blocking (Preventing Nonspecific Binding): To minimize background noise and prevent unwanted interactions, a blocking solution such as bovine serum albumin (BSA) or non-fat milk is added. This step is crucial for enhancing assay specificity by reducing nonspecific binding of antibodies to the plate.
3. Detection (Antibody Binding and Enzyme Labeling): The sample containing the target protein is introduced, followed by incubation with a primary antibody that specifically binds to the molecule of interest. A secondary antibody conjugated to an enzyme (such as horseradish peroxidase or alkaline phosphatase) is then added to facilitate detection.
4. Signal Development and Quantification: A substrate specific to the enzyme is introduced, triggering a colorimetric, fluorescent, or chemiluminescent reaction. The intensity of the signal correlates with the concentration of the target molecule and is measured using a spectrophotometer or plate reader.
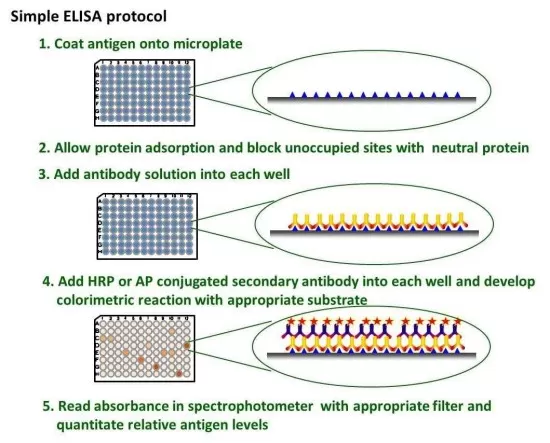
Workflow of ELISA
Advantages of ELISA
ELISA is widely recognized for its high sensitivity, allowing detection of proteins at concentrations as low as picograms per milliliter (pg/mL). The assay’s ability to process multiple samples simultaneously makes it particularly advantageous for large-scale screening applications, such as drug discovery, vaccine development, and clinical diagnostics. The quantitative nature of ELISA ensures accurate and reproducible measurements, which is essential in clinical and research settings. Furthermore, its automation-friendly format enables integration into high-throughput workflows, minimizing manual intervention and increasing efficiency.
Limitations of ELISA
Despite its strengths, ELISA is not without limitations. One of the key drawbacks is its susceptibility to interference, which can arise from sample contamination, improper washing steps, or cross-reactivity between antibodies. Additionally, ELISA provides limited structural information, meaning it cannot determine the molecular weight or isoforms of a target protein. This limitation makes it unsuitable for distinguishing between closely related protein variants. Furthermore, the assay requires high-quality antibodies to ensure specificity. Poor antibody selection can lead to false positives or false negatives, impacting the reliability of results. Researchers must also ensure that sample concentrations fall within the dynamic range of the assay, as excessively high or low concentrations may lead to inaccurate quantification.
What is Western Blot?
Western Blot is a molecular biology technique used for detecting and characterizing specific proteins within a complex mixture. Unlike ELISA, which is primarily used for quantification, Western Blot provides structural and qualitative information, including protein size, isoforms, and post-translational modifications. This makes it an invaluable tool for confirming protein identity and detecting modifications such as phosphorylation, glycosylation, or ubiquitination.
Step-by-Step Process of Western Blot
Western Blot involves multiple steps, each requiring meticulous execution to ensure accurate results.
1. Protein Separation via SDS-PAGE: The first step involves separating proteins by molecular weight using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). This technique ensures that proteins migrate based on their size, enabling resolution of individual proteins within a complex sample.
2. Protein Transfer to a Membrane: Once proteins are separated, they are transferred onto a polyvinylidene difluoride (PVDF) or nitrocellulose membrane. This step is essential for immobilizing proteins on a stable surface where antibody-based detection can occur.
3. Blocking to Reduce Nonspecific Binding: Similar to ELISA, a blocking solution is applied to prevent unwanted interactions and reduce background noise.
4. Antibody Incubation and Detection: The membrane is incubated with a primary antibody specific to the target protein, followed by a secondary antibody conjugated to an enzyme. Detection is achieved through chemiluminescence, fluorescence, or colorimetric methods, producing visible protein bands.
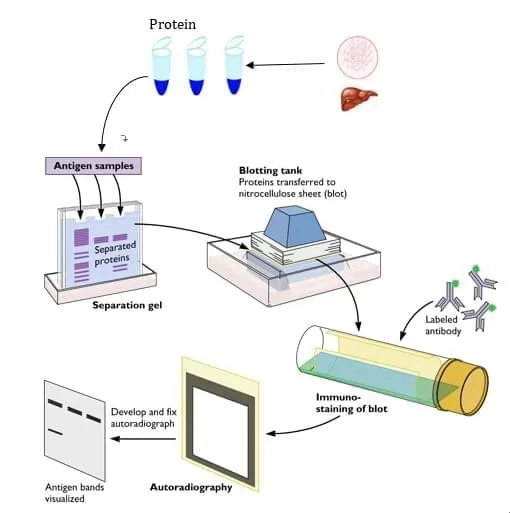
Workflow of WB
Advantages of Western Blot
Western Blot is highly specific, making it an excellent choice for confirming protein identity and minimizing false positives. One of its key advantages is its ability to provide molecular weight information, allowing researchers to verify the integrity and size of the detected protein. Additionally, it is uniquely suited for analyzing protein modifications, which are critical in studies of cell signaling and disease mechanisms.
Limitations of Western Blot
While Western Blot offers high specificity, it is less sensitive than ELISA, typically detecting proteins at concentrations in the nanogram per milliliter (ng/mL) range. The process is also labor-intensive and time-consuming, requiring 1–2 days to complete. Furthermore, the technique is semi-quantitative, meaning it is not as precise as ELISA for absolute quantification. Reproducibility can be a challenge due to variability in sample loading, transfer efficiency, and antibody performance.
ELISA vs. Western Blot: Key Differences and Best Use Cases
|
Feature |
ELISA |
Western Blot |
|
Best for |
High-throughput quantification |
Protein characterization and validation |
|
Sensitivity |
High (pg/mL) |
Moderate (ng/mL) |
|
Molecular Weight Information |
No |
Yes |
|
Post-Translational Modifications |
No |
Yes |
|
Time Required |
4–6 hours |
1–2 days |
When deciding between ELISA and Western Blot, researchers should consider their specific experimental objectives. If the goal is high-throughput quantification, ELISA is the best choice. However, if molecular weight confirmation or detection of post-translational modifications is required, Western Blot is the superior technique.
In many cases, both methods are used in combination—ELISA for initial screening and Western Blot for validation—providing a comprehensive analysis of the target protein.
Read more
- LC-MS VS GC-MS: What's the Difference
- LC vs. HPLC vs. UHPLC: Tracing the Evolution of Chromatographic Techniques
- Mastering Chromatography: Everything You Need to Know
- DIA Proteomics vs DDA Proteomics: A Comprehensive Comparison
- Multi-Omics Association Analysis Series
- Omics Data Processing Series
- Omics Data Analysis Series


