DIA Proteomics Unveils EMPA's Mechanisms in Heart Failure Regulation
Role of Empagliflozin (EMPA) in Heart Failure and Senescence
Heart failure (HF) is a chronic, progressive condition in which the heart muscle weakens or stiffens, impairing its ability to pump blood effectively to meet the body's demands. This leads to reduced oxygen and nutrient delivery, causing symptoms such as fatigue, shortness of breath, and fluid buildup (edema) in the lungs, legs, and abdomen. The main types of heart failure are categorized based on ejection fraction (EF), a measure of how much blood the heart pumps out with each contraction.
HFpEF, also known as diastolic heart failure, occurs when the heart's ejection fraction is normal (usually ≥50%), meaning the heart pumps a sufficient proportion of blood out of the left ventricle with each beat. However, HFpEF is characterized by diastolic dysfunction, where the heart muscle becomes stiff and less elastic, reducing its ability to relax and fill properly during diastole (the phase when the heart chambers refill with blood). As a result, the heart cannot accommodate enough blood between beats, leading to increased pressure in the left atrium and pulmonary veins. This leads to symptoms like shortness of breath, fatigue, and swelling, as blood flow is restricted despite the heart's preserved pumping strength. HFpEF is particularly common among older adults, especially those with conditions like hypertension, obesity, and diabetes. HFpEF is a significant challenge in cardiology today due to the limited availability of effective therapies. Its incidence may reach up to 8% among individuals aged 65 and older, and even exceeds 10% in those aged 75 and above.
Empagliflozin (EMPA) is a medication originally developed to manage type 2 diabetes by reducing blood glucose levels. It belongs to the class of sodium-glucose cotransporter 2 (SGLT2) inhibitors, which work by blocking the reabsorption of glucose in the kidneys, promoting its excretion through urine. This mechanism lowers blood sugar levels and reduces the risk of hyperglycemia. Beyond its glucose-lowering effects, empagliflozin has shown substantial benefits for cardiovascular health, making it a groundbreaking therapy for managing heart disease. Several mechanisms have been proposed to explain the cardiovascular benefits of empagliflozin (EMPA), and anti-senescence effects have been observed in kidney tissue. However, the exact mechanisms through which EMPA provides protection against HFpEF and its potential role in cardiac aging remain unclear.
DIA Quantitative Proteomics: A Powerful Tool
Proteomics is the large-scale study of proteins, the essential molecules that perform most functions in cells and tissues. By identifying, quantifying, and analyzing proteins within a sample, proteomics provides insights into biological processes, disease mechanisms, and cellular responses. Techniques like mass spectrometry (MS) are central to proteomics, allowing for the high-throughput analysis of complex samples, such as tissues, blood, or cell lysates. Through proteomics, researchers can explore protein expression, modifications, interactions, and structures, making it a valuable tool for biomarker discovery, drug development, and personalized medicine. This field plays a crucial role in multiomics research by integrating proteomic data with genomic, transcriptomic, and metabolomic information to uncover comprehensive insights into cellular biology and disease pathology.
Data-Independent Acquisition (DIA) quantitative proteomics is a modern MS-based approach that overcomes limitations of traditional proteomics by providing a more comprehensive and reproducible protein analysis. Unlike Data-Dependent Acquisition (DDA), which selectively targets a subset of peptides, DIA fragments all detectable peptides within specific mass-to-charge (m/z) ranges, ensuring an unbiased and complete view of the proteome. This method improves sensitivity and quantitative accuracy, especially for low-abundance proteins that are often missed in DDA. Using spectral libraries for data interpretation, DIA enables confident identification and quantification of proteins across complex samples. Its ability to provide consistent, high-coverage data makes DIA proteomics particularly useful for biomarker discovery, disease research, and longitudinal studies where reproducibility is essential.
DIA Proteomics Empower Mechanistic Insights into EMPA's Role in Heart Failure
To explore the mechanisms underlying EMPA's beneficial effects on HFpEF and its potential role in regulating anti-aging pathways, a research team recently published a study in Cardiovascular Diabetology titled, Empagliflozin Protects Against Heart Failure with Preserved Ejection Fraction Partly by Inhibiting the Senescence-Associated STAT1–STING Axis. In this investigation, the researchers established a mouse model of HFpEF, subsequently administering EMPA to assess its therapeutic effects. They then performed DIA quantitative proteomics analysis on samples from the HFpEF model, EMPA-treated mice, and healthy control mice.
Using DIA quantitative proteomics, a total of 5,702 proteins were identified across the three groups of mouse hearts. Differentially expressed proteins (DEPs) were selected based on a |log2(fold change)| greater than 0.26 and a P value of less than 0.05. Among these, 310 DEPs were identified in HFpEF hearts compared to control hearts, comprising 177 upregulated and 133 downregulated proteins. Additionally, 173 DEPs were identified between the HFpEF and EMPA groups, with 99 proteins showing upregulation and 74 downregulation. Furthermore, K-means cluster analysis categorized all differentially expressed proteins (DEPs) into ten groups based on their expression patterns. Most coregulated proteins were concentrated in clusters 1, 2, 6, and 10, which exhibited U-shaped or reverse U-shaped profiles.
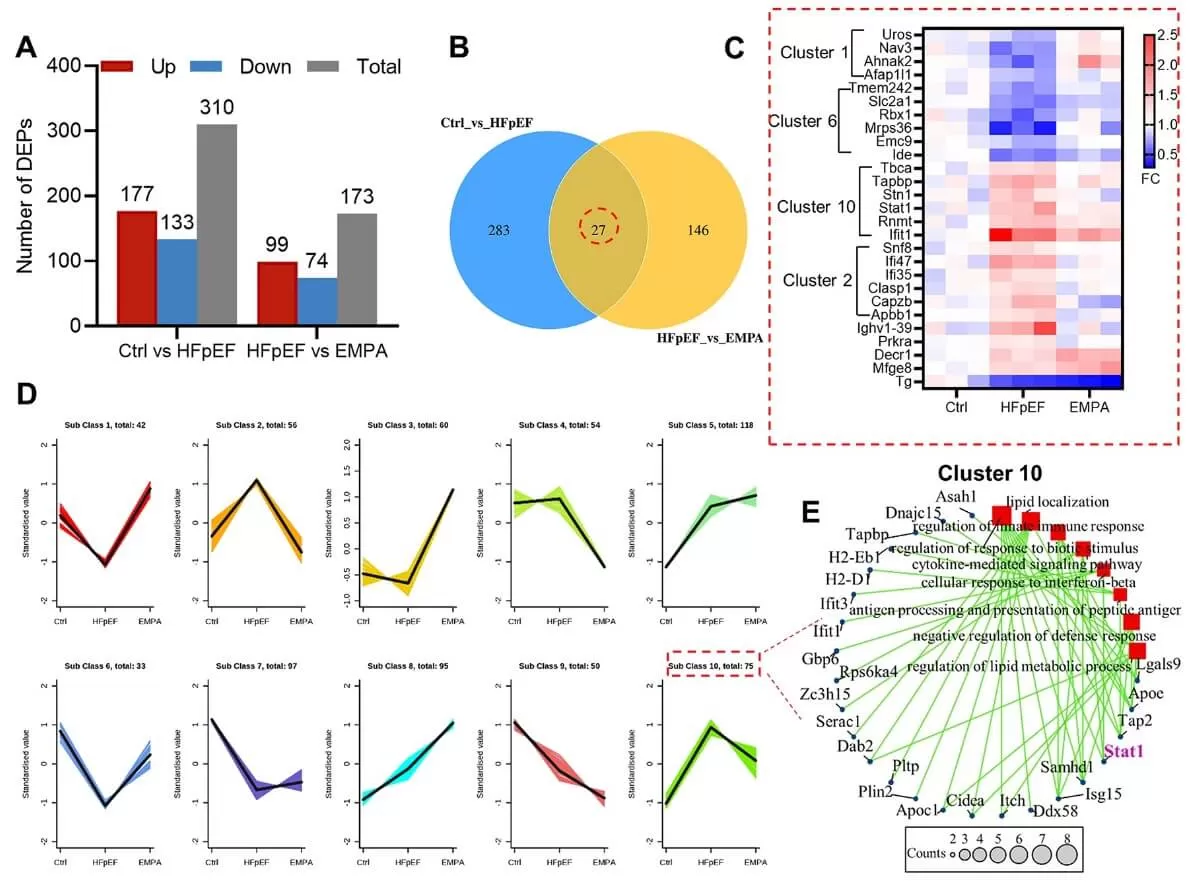
To investigate the key factors contributing to the pathogenesis of HFpEF, the authors conducted enrichment analyses of Gene Ontology (GO) biological processes and KEGG pathways for the differentially expressed proteins (DEPs) identified between the control and HFpEF groups, as well as those between the HFpEF and EMPA groups. The results showed that the regulation of immune system processes was enriched in all groups and the interferon response genes (STAT1, Ifit1, Ifi35 and Ifi47) were upregulated in HFpEF mice but downregulated after EMPA administration. Further protein interaction networks verified STAT1 as the hub transcription factor during pathological changes in HFpEF mice.
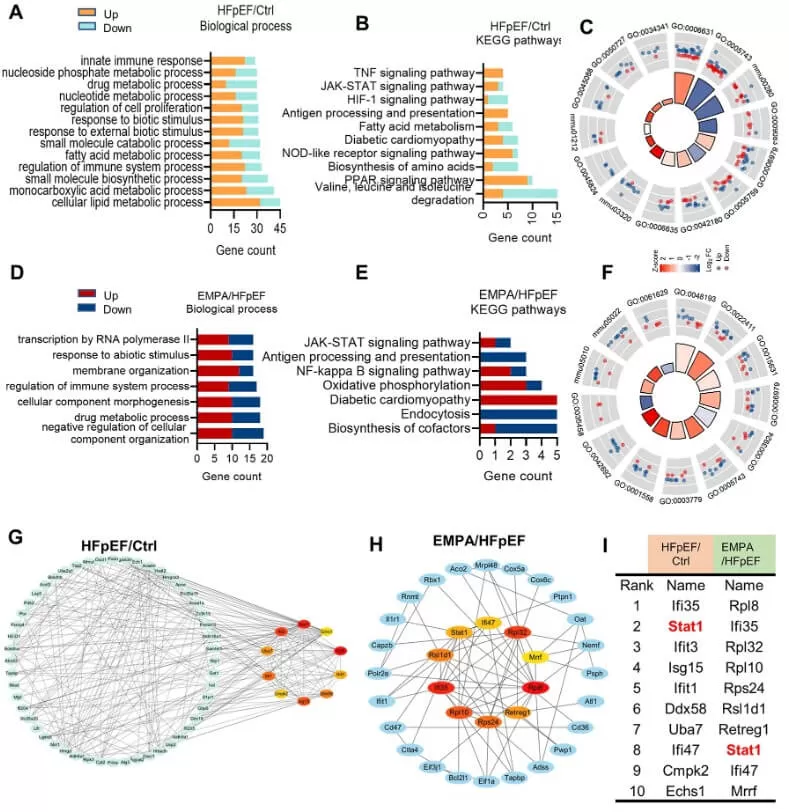
Through the DIA quantitative proteomics analysis, the authors proposed that EMPA may protect against cardiac deterioration in HFpEF by partially mitigating the immune response through the regulation of STAT1 activity.
DIA Proteomics Enhance Understanding of EMPA's Role in Aging
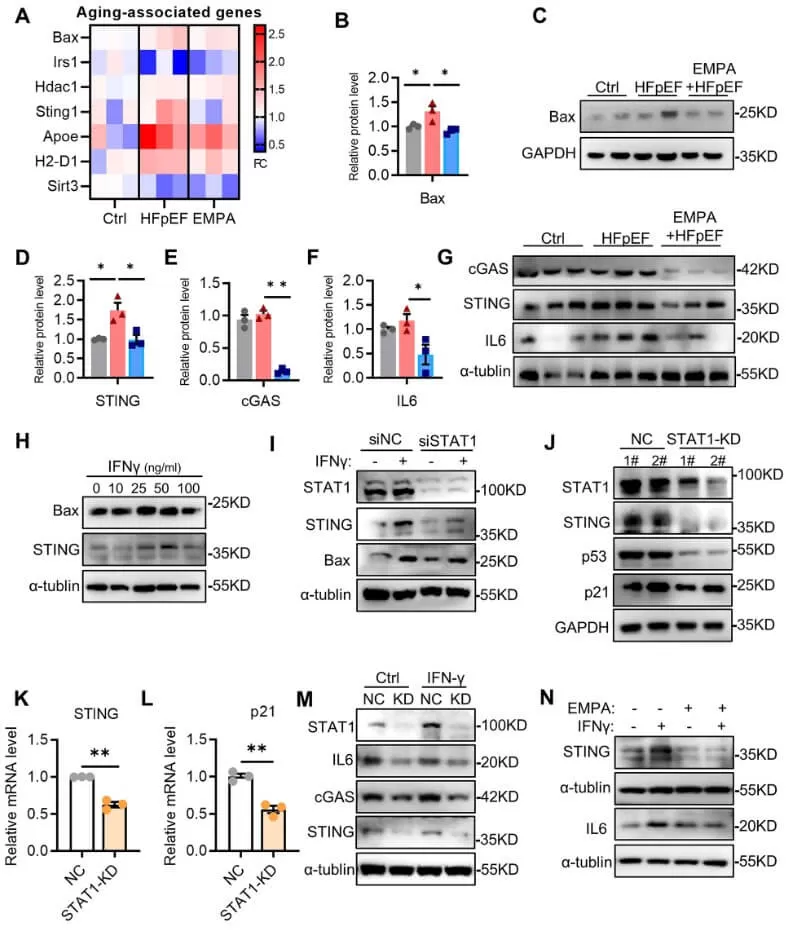
The authors also discovered that EMPA suppresses IFN-γ-induced cardiomyocyte senescence by inhibiting STAT1 activation. To elucidate the detailed regulatory mechanisms, they screened aging-associated genes from the proteomic data, revealing that EMPA treatment in mice inhibited the increased expression of Bax, Sting, ApoE, and H2-D1 genes in HFpEF hearts. Immunoblotting further confirmed that the protein levels of Bax, cGAS, STING, and IL-6 were elevated in HFpEF mice compared to control mice, and these changes were attenuated in EMPA-treated mice. Notably, the cGAS–STING pathway is a significant driver of aging-related inflammation. To investigate whether STAT1 regulates STING expression during aging, the authors established a stable cell line with STAT1 knockdown (KD). Consistent with the findings from STAT1 inhibition using siRNA, STING expression was reduced in STAT1-KD cells compared to normal control cells, and the senescence markers p53 and p21 were also diminished following STAT1 knockdown. Thus, the authors concluded that inhibiting STAT1 significantly reduces cellular senescence, likely by regulating STING expression.
Constructing Regulatory Networks of EMPA in Heart Failure and Aging
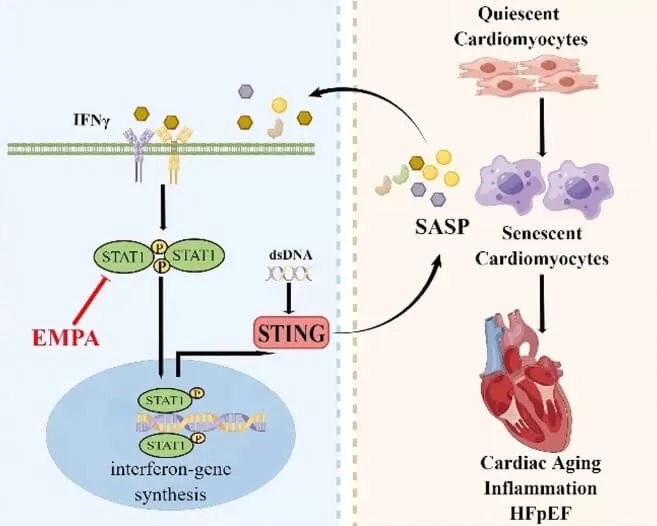
Finally, this research revealed that EMPA mitigates cardiac inflammation and aging in HFpEF mice by inhibiting STAT1 activation. The STAT1–STING axis may act as a pivotal mechanism in the pathogenesis of HFpEF, especially under inflammatory and aging conditions.
MetwareBio: Your Trusted Partner in Proteomics and Multiomics Research
MetwareBio provided DIA quantitative proteomics services for this research, enabling the authors to pinpoint two critical proteins, STAT1 and STING, within the regulatory networks associated with HFpEF. This collaboration highlights MetwareBio's commitment to advancing scientific research through high-quality proteomic analysis. As a professional contract research organization (CRO) specializing in proteomics and multiomics, MetwareBio leverages state-of-the-art technologies and methodologies to deliver comprehensive insights into complex biological systems. By integrating proteomic data with other omics approaches, MetwareBio supports researchers in uncovering novel biomarkers and therapeutic targets, ultimately facilitating the development of innovative treatments for various diseases. The successful identification of STAT1 and STING underscores the importance of precise proteomic profiling in understanding the intricate mechanisms underlying cardiac health and disease.
If you have any research needs or inquiries regarding our services, please do not hesitate to contact us. Our dedicated team of experts is ready to assist you in achieving your scientific goals. At MetwareBio, we are committed to providing personalized support and innovative solutions tailored to your specific research requirements. Let us partner with you in advancing your research and discovering new possibilities in proteomics and multiomics.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


