Decoding Life's Molecular Dialogue: An In-Depth Exploration of Protein-Protein Interactions (PPI)
Proteins are the fundamental building blocks of life, carrying out essential biological functions within cells. However, proteins rarely act alone. Instead, they engage in intricate interactions with other proteins, forming a highly dynamic and complex network known as Protein-Protein Interactions (PPI). These interactions are pivotal in regulating biological processes, ensuring cellular communication, and maintaining homeostasis.
From fundamental cellular functions such as DNA replication and metabolism to critical physiological processes like immune responses and signal transduction, PPI networks dictate the functional landscape of living organisms. The disruption of these networks can lead to severe pathological conditions, including cancer, neurodegenerative disorders, and infectious diseases. As a result, understanding PPIs is not only fundamental to deciphering biological mechanisms but also holds immense potential for therapeutic innovations, particularly in drug discovery.
This article provides an extensive exploration of PPI, discussing its nature, experimental and computational approaches for studying PPIs, the significance of PPI networks in multi-omics research, current challenges in the field, and future directions.
Understanding the Nature of Protein-Protein Interactions
PPIs can be described as molecular dialogues, where proteins communicate and cooperate to execute biological functions. Unlike static molecular interactions, PPIs are dynamic, meaning that their occurrence, strength, and duration are influenced by various intracellular and extracellular factors. These include environmental conditions, post-translational modifications (such as phosphorylation), and protein concentration gradients.
The nature of PPIs can be classified based on their stability, duration, and functional role within the cell. Some interactions are highly stable and persist throughout the lifetime of a protein complex, while others are transient, occurring only when specific cellular events require them.
Stable Protein Complexes
Some proteins form highly stable interactions that create permanent protein complexes, essential for cellular integrity and function. Examples include ribosomes, proteasomes, and cytoskeletal structures like actin filaments. These complexes provide structural support and facilitate crucial biological processes, such as protein degradation and intracellular transport.
Transient Interactions
In contrast to stable interactions, transient PPIs occur for short durations and often play a role in signaling pathways and regulatory mechanisms. For instance, kinases interact with their substrates momentarily to phosphorylate them, initiating downstream signaling cascades. Similarly, transcription factors transiently bind to DNA-binding proteins to regulate gene expression dynamically. These fleeting interactions allow cells to rapidly respond to internal and external stimuli, ensuring adaptability in a changing environment.
Illustration of PPI
Competitive Interactions
PPIs are not always cooperative; in some cases, proteins compete for binding sites, leading to regulatory effects. An example is the interaction between transcription factors and their inhibitory proteins, where competitive binding determines whether a gene is activated or repressed. Such regulatory interactions are vital for maintaining cellular balance and preventing aberrant gene expression that could lead to diseases like cancer.
Revolutionary Techniques for Studying PPI
Over the years, significant technological advancements have transformed the study of PPIs, enabling researchers to decipher complex interaction networks at an unprecedented scale. These methodologies can be broadly categorized into experimental techniques and computational approaches.
Experimental Techniques for Detecting PPIs
Yeast Two-Hybrid (Y2H) System: The Yeast Two-Hybrid system is one of the most established methods for detecting binary protein interactions. By genetically engineering yeast cells to express two interacting proteins fused with a reporter system, researchers can identify direct interactions through changes in cellular phenotype. This method is particularly useful for large-scale screening but has limitations, such as high false-positive and false-negative rates.
Co-Immunoprecipitation (Co-IP): Co-IP is a widely used technique that validates PPIs in their natural cellular context. By using specific antibodies to pull down a target protein along with its interacting partners, researchers can confirm interactions that occur in vivo. However, Co-IP requires highly specific antibodies, and its success depends on the stability of the protein complex being studied.
Fluorescence Resonance Energy Transfer (FRET): FRET is an advanced method that enables real-time observation of protein interactions within living cells. By tagging proteins with fluorescent markers that transfer energy upon close proximity, researchers can visualize and quantify interactions dynamically. This technique provides high spatial and temporal resolution but requires sophisticated imaging systems and is limited to specific interaction distances.
Computational Approaches and AI Innovations in PPI Research
The advent of artificial intelligence has revolutionized PPI research, allowing for accurate prediction and modeling of protein complexes. Deep learning models such as AlphaFold-Multimer have demonstrated remarkable success in predicting protein complex structures with atomic-level accuracy.
Another breakthrough is MAPE-PPI, a computational framework introduced at the 2024 ICLR conference. By integrating microenvironmental awareness into PPI predictions, MAPE-PPI has significantly enhanced accuracy, outperforming traditional methods by 27%. These computational tools are accelerating drug discovery efforts by identifying potential protein targets and interaction hotspots for therapeutic intervention.
PPI Networks: A Gateway to Multi-Omics Research
A Protein-Protein Interaction Network (PPI Network) is a graphical representation of protein interactions, where proteins are depicted as nodes and their interactions as edges. These networks encapsulate direct physical interactions, signaling relationships, and regulatory dependencies, providing a holistic view of cellular function.
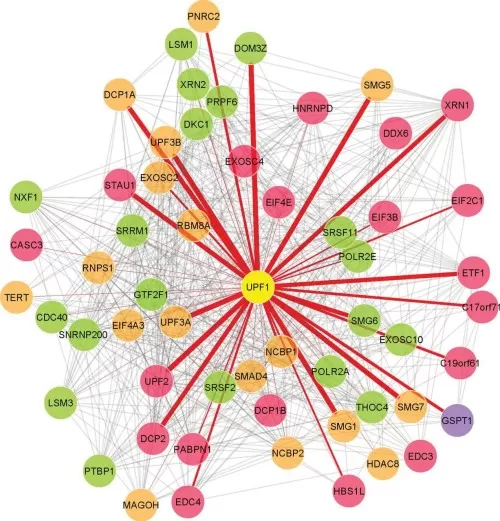
The PPI network
Applications of PPI Networks in Systems Biology
PPI networks play a crucial role in multi-omics research, integrating genomics, transcriptomics, and proteomics data to uncover disease mechanisms and biological pathways.
- Identification of Key Regulatory Proteins: By analyzing the connectivity of proteins within a network, researchers can pinpoint hub proteins—key regulators that orchestrate biological functions. Many of these hub proteins serve as biomarkers for diseases, including cancer and neurodegenerative disorders.
- Discovery of Functional Modules: Within PPI networks, proteins tend to cluster into functional modules that execute specialized biological tasks. Identifying these modules provides insights into cellular mechanisms, such as metabolic pathways and immune responses.
- Disease Mechanism Investigation: Mapping PPI networks specific to disease conditions can reveal novel drug targets and therapeutic strategies. For instance, alterations in PPIs have been implicated in cancer progression, leading to the development of targeted therapies such as kinase inhibitors.
Building a PPI Network: Key Databases and Tools
- STRING Database: STRING is a widely used resource that catalogs known and predicted protein interactions, encompassing over 5,000 species and 30 billion interaction data points.
- Cytoscape Software: Cytoscape is a powerful tool for visualizing and analyzing PPI networks, providing plugins like cytoHubba for identifying essential regulatory nodes.
Challenges and Future Prospects in PPI Research
Despite technological advancements, several hurdles remain in PPI research. The detection of transient and weak interactions is particularly challenging due to their fleeting nature. Additionally, computational models, while improving, still face difficulties in accurately predicting interactions in complex environments, such as membrane-bound proteins.
To overcome these limitations, researchers are integrating multi-omics data with single-cell and spatial transcriptomics, aiming to construct cell-type-specific PPI networks. Additionally, AI-driven dynamic simulations, such as the Virtual Cell platform reported in Nature (2025), are emerging as promising tools for real-time modeling of PPIs in physiological conditions.
With the convergence of big data, AI, and high-throughput screening, the future of PPI research is poised to redefine drug discovery and personalized medicine. Mastering these molecular interactions will be key to unlocking novel therapies and advancing our understanding of life's intricate biological processes.
Conclusion: The Dawn of a New Era in PPI Research
PPI research is at the forefront of modern molecular biology, offering unprecedented insights into cellular function and disease mechanisms. As scientific methodologies evolve, the potential for translating PPI knowledge into precision medicine and targeted therapies grows exponentially. The next decade will witness a transformation in how we decode protein interactions—ushering in an era where PPI-guided therapeutics redefine biomedical innovation.


