Decoding Protein Crosslinking: From Basic Concepts to Advanced Methods
In the realm of biochemistry, proteins play an incredibly crucial role. They not only constitute the structural components of cells but also participate in nearly all biochemical reactions. The functionality of proteins largely depends on their three-dimensional structure, which can be stabilized or altered through a process known as "cross-linking." Protein cross-linking is an important concept in biochemistry and molecular biology, involving interactions between protein molecules. Today, this blog will introduce two types of protein cross-linking: exogenous cross-linking and endogenous cross-linking, along with their roles and distinctions in biological systems.
What is Protein Cross-Linking?
Protein cross-linking refers to the process of covalently linking two or more protein molecules together. This connection can occur between amino acid residues within the same protein molecule or between different protein molecules. Cross-linking can occur naturally or be induced artificially using cross-linking agents.
Exogenous Protein Cross-Linking
Exogenous protein cross-linking refers to the process of promoting connections between protein molecules during protein synthesis by adding external substances, such as cross-linking agents. This type of cross-linking typically occurs at specific amino acid residues in proteins, such as lysine, cysteine, or tyrosine. For instance, cisplatin, a common exogenous cross-linking agent used in chemotherapy, forms interstrand cross-links with DNA, thereby obstructing the processes of DNA replication and transcription. Additionally, exogenous cross-linking can also be achieved through photoreactive cross-linking groups, which are often employed to study protein interactions. Exogenous protein cross-linking has limited controllability, which can lead to cellular damage or even cell death. Consequently, while this type of cross-linking holds potential applications in medical treatments, it must be used with caution to avoid unnecessary side effects.
Key Features of Exogenous Cross-Linking:
- Controllability: By selecting specific cross-linking agents and reaction conditions, researchers can precisely control the location and extent of cross-linking.
- Diversity: A variety of cross-linking agents can be utilized to meet different experimental needs.
- Wide Applications: Exogenous cross-linking is widely applied in fields such as biotechnology, drug development, and materials science.
Applications for Exogenous Cross-Linking:
- Biosensors: Cross-linking techniques can be used to immobilize specific biomolecules on sensor surfaces, enhancing detection sensitivity and selectivity.
- Drug Delivery: Cross-linked proteins can serve as drug carriers, improving the stability and bioavailability of pharmaceuticals.
Endogenous Protein Cross-Linking
In contrast to exogenous cross-linking, endogenous protein cross-linking refers to the naturally occurring cross-linking processes within organisms. This type of cross-linking is typically catalyzed by intracellular enzymes, such as transglutaminase, which facilitates the formation of isopeptide bonds between protein molecules. Endogenous protein cross-linking has complex and diverse effects on cells. On one hand, it can be part of the cellular stress response, helping cells cope with environmental pressures like oxidative stress and temperature fluctuations. On the other hand, excessive or abnormal endogenous cross-linking may lead to the onset of diseases, such as progeria. Thus, studying the regulatory mechanisms of endogenous protein cross-linking is crucial for understanding both physiological and pathological states of cells.
Key Features of Endogenous Cross-Linking:
- Natural Occurrence: This process is a chemical reaction that happens naturally within the organism without the need for additional cross-linking agents.
- Precision: Endogenous cross-linking typically occurs at specific amino acid residues and is catalyzed by designated enzymes.
- Biological Significance: Endogenous cross-linking plays a vital role in stabilizing cell structures, signal transduction, and protein degradation.
Biological Significance of Endogenous Cross-Linking:
- Cytoskeleton: Within cells, microtubules and actin filaments form stable cytoskeletal structures through endogenous cross-linking.
- Coagulation: In response to injury, fibrinogen in the blood undergoes endogenous cross-linking to form fibrin, promoting blood clotting.
Differences between Exogenous and Endogenous Cross-Linking
- Mechanism of Occurrence: Exogenous cross-linking involves the participation of external substances, whereas endogenous cross-linking occurs naturally within the organism.
- Level of Control: Exogenous cross-linking can be controlled by selecting specific cross-linking agents and conditions, while endogenous cross-linking is regulated by the internal environment of the organism.
- Application Fields: Exogenous cross-linking is more commonly found in scientific research and industrial applications, whereas endogenous cross-linking plays a crucial role in the physiological processes of living organisms.
Methods for Studying Protein Cross-Linking
- Mass Spectrometry (Crosslinking Mass Spectrometry - XL-MS): XL-MS is a powerful technique for identifying and characterizing protein interactions through crosslinking. By treating proteins with specific crosslinkers, peptides formed can be isolated and analyzed via mass spectrometry, allowing researchers to determine the identity and location of crosslinked residues. This method provides insights into protein conformation and interaction networks and can reveal distance constraints between amino acids, offering a dynamic view of protein structures.
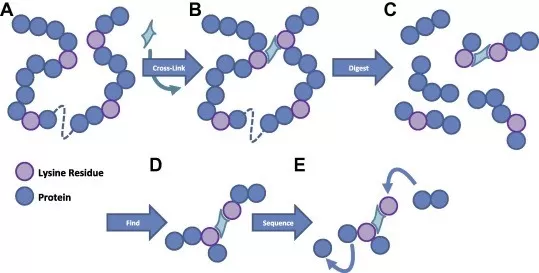
- Biochemical Methods (Western Blotting and ELISA): This approach involves the use of homobifunctional or heterobifunctional crosslinkers that covalently bond proteins together. The resulting crosslinked complexes can then be separated using techniques like SDS-PAGE, followed by identification through Western blotting or Enzyme-linked immunosorbent assays (ELISA). This method is particularly useful for stabilizing transient interactions and assessing the oligomeric state of proteins, facilitating the study of complex formations in various biological contexts.
- Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR spectroscopy is utilized to investigate the structure and dynamics of proteins in solution. By labeling crosslinked residues with isotopes, researchers can observe shifts in resonances that indicate conformational changes or interactions resulting from crosslinking. This method provides detailed information about the three-dimensional structure of proteins and their complexes, helping to elucidate mechanisms of action and interaction.
- Cryo-Electron Microscopy (Cryo-EM): Cryo-EM enables the visualization of protein complexes at near-atomic resolution without the need for crystallization. This technique involves rapidly freezing samples to preserve their native structure and then imaging them using electron microscopy. Cryo-EM can reveal the spatial arrangement of crosslinked proteins in large complexes, offering insights into their structural organization and functional roles within cellular processes.
- X-ray Crystallography: This technique provides high-resolution structural data for proteins and protein complexes, including those that are crosslinked. By crystallizing the crosslinked proteins and subjecting them to X-ray diffraction, researchers can determine the arrangement of atoms in the crystal, yielding detailed information about crosslinking sites and the overall conformation of the proteins. X-ray crystallography is particularly valuable for elucidating static structures, which can help in understanding the functional implications of crosslinking.
- Fluorescence Resonance Energy Transfer (FRET): FRET is a sensitive technique for studying protein-protein interactions in live cells. By tagging proteins with donor and acceptor fluorophores, researchers can monitor energy transfer between the tags when the proteins come into close proximity, often as a result of crosslinking. Changes in FRET efficiency can indicate dynamic interactions and conformational changes, providing real-time insights into protein behavior and interactions within their native environments.
References
1. Holding A. N. (2015). XL-MS: Protein cross-linking coupled with mass spectrometry. Methods (San Diego, Calif.), 89, 54–63. https://doi.org/10.1016/j.ymeth.2015.06.010
2. Wang JH, Tang YL, Gong Z, Jain R, Xiao F, Zhou Y, Tan D, Li Q, Huang N, Liu SQ, Ye K, Tang C, Dong MQ, Lei X. Characterization of protein unfolding by fast cross-linking mass spectrometry using di-ortho-phthalaldehyde cross-linkers. Nat Commun. 2022 Mar 18;13(1):1468. doi: 10.1038/s41467-022-28879-4. PMID: 35304446; PMCID: PMC8933431.
3. Guo AD, Yan KN, Hu H, Zhai L, Hu TF, Su H, Chi Y, Zha J, Xu Y, Zhao D, Lu X, Xu YJ, Zhang J, Tan M, Chen XH. Spatiotemporal and global profiling of DNA-protein interactions enables discovery of low-affinity transcription factors. Nat Chem. 2023 Jun;15(6):803-814. doi: 10.1038/s41557-023-01196-z. Epub 2023 Apr 27. PMID: 37106095.
4. Long M, Zheng N, Zhang Z, Gao L, Wang Y, Xia X. [Mechanisms and applications of enzyme-catalyzed protein cross-linking]. Sheng Wu Gong Cheng Xue Bao. 2022 Jul 25;38(7):2499-2512. Chinese. doi: 10.13345/j.cjb.210875. PMID: 35871620.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


