Unlocking the Future of CKD Diagnosis: Metabolomics and AI-Driven Biomarker Discovery
We are proud to have co-authored a recent study published in Chinese Chemical Letters, titled "Metabolome profiling by widely-targeted metabolomics and biomarker panel selection using machine-learning for patients in different stages of chronic kidney disease." Conducted in collaboration with leading researchers, this study employs widely-targeted metabolomics (WT-Met) to profile the plasma metabolome of patients at different stages of chronic kidney disease (CKD). By integrating machine-learning techniques, the study identifies a panel of seven metabolites with high diagnostic potential, achieving an impressive average AUC of 0.99. This work underscores the power of metabolomics in uncovering disease-specific metabolic alterations and advancing precision medicine for CKD management.
Understanding Metabolomics in CKD Progression
Chronic kidney disease (CKD) is a prevalent global health issue associated with high morbidity and mortality. Traditional biomarkers such as serum creatinine and the estimated glomerular filtration rate (eGFR) have been used for decades but fail to capture the full complexity of kidney dysfunction. The integration of metabolomics offers a promising avenue for identifying more accurate and dynamic biomarkers that reflect metabolic alterations associated with CKD progression.
Metabolomics provides a comprehensive view of biochemical changes occurring within the body. The study applies widely-targeted metabolomics (WT-Met), a hybrid approach combining targeted and untargeted metabolomics, to achieve large-scale metabolite identification and quantification. This enables researchers to explore the metabolic landscape of CKD with unprecedented detail and sensitivity.
 Schematic illustration of the study design. (B) The workflow of the WT-Met approach._1741772570_WNo_792d665.webp)
(A) Schematic illustration of the study design. (B) The workflow of the WT-Met approach.
Comprehensive Plasma Metabolome Analysis
Using liquid chromatography-mass spectrometry (LC-MS)-based WT-Met, the study analyzed plasma samples from 21 healthy individuals and 62 CKD patients categorized into different disease stages (22 in stages 1–3, 20 in stage 4, and 20 in stage 5). A total of 1,431 metabolites were identified, of which 539 were endogenous compounds relevant to CKD progression. Notably, 399 metabolites showed significant alterations across different disease stages, highlighting the metabolic disruptions associated with CKD.
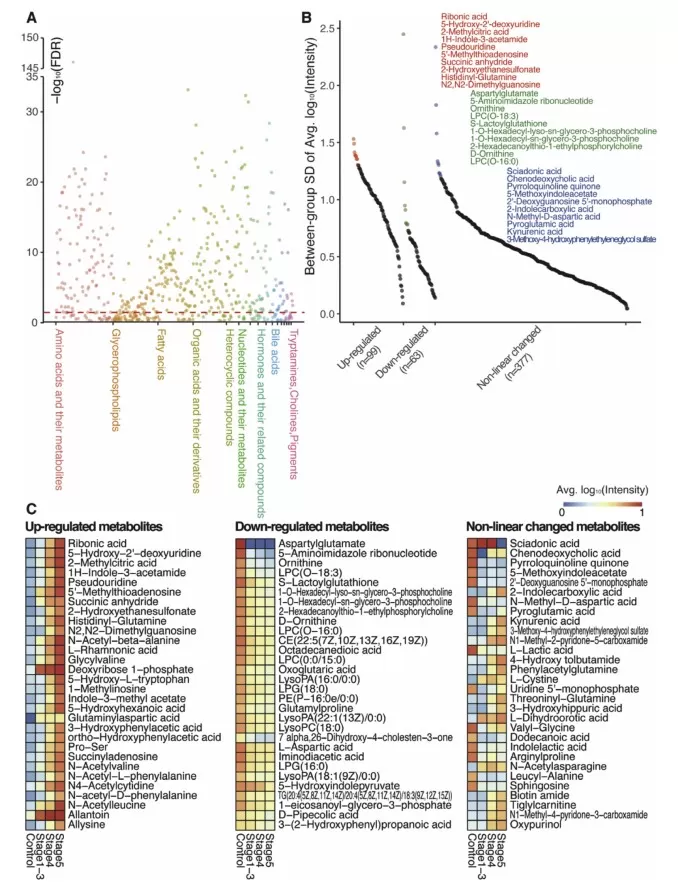
Significantly altered metabolites across the progression of CKD.
Identification of Key Metabolic Pathways and Metabolite Clustering
Among the altered metabolites, amino acids, nucleotides, and organic acids emerged as the most significantly affected categories. Further clustering analysis grouped the metabolites into six distinct clusters based on their changing trends throughout CKD progression. Some metabolites exhibited a steady increase or decrease, while others followed non-linear patterns, reflecting the dynamic metabolic shifts occurring in CKD patients. KEGG pathway analysis identified four significantly altered metabolic pathways related to amino acid metabolism, reinforcing the importance of these biochemical changes in CKD development.
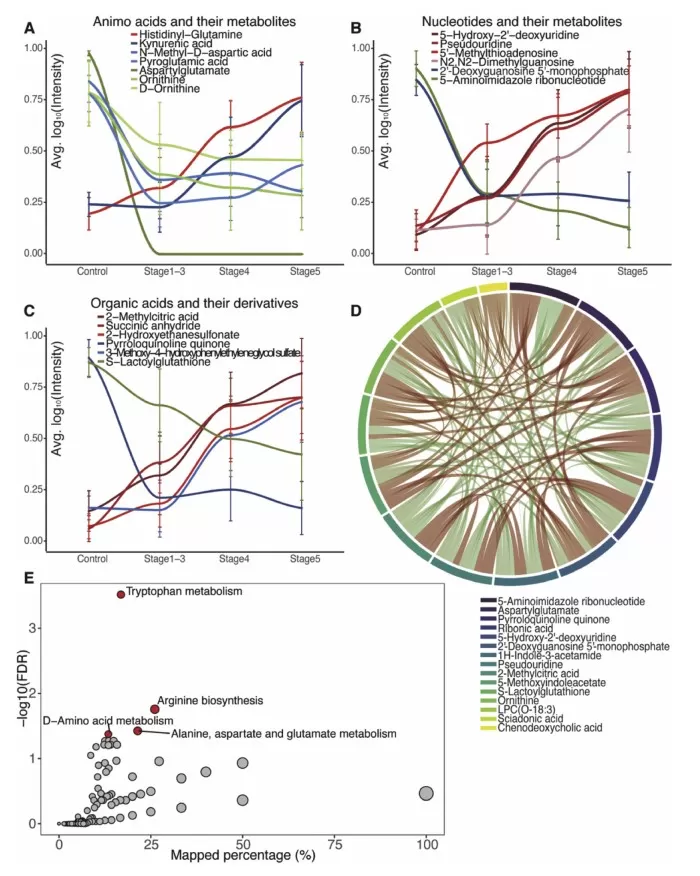
Altered groups of metabolites and pathways during CKD progression.
Machine Learning-Based Biomarker Panel Selection
The study employed machine-learning techniques to select an optimal biomarker panel for CKD staging. A seven-metabolite panel was identified using a random forest algorithm, achieving an exceptional classification performance with an average AUC of 0.99. These biomarkers include 5-aminoimidazole ribonucleotide, 5′-methylthioadenosine, sciadonic acid, allysine, 5-methoxyindoleacetate, deoxyribose 1-phosphate, and 2-hydroxyethanesulfonate, each with distinct roles in metabolic pathways relevant to CKD progression. Notably, 5-aminoimidazole ribonucleotide is crucial in purine metabolism, while 5′-methylthioadenosine is involved in polyamine biosynthesis, both exhibiting distinct shifts in concentration with CKD severity.
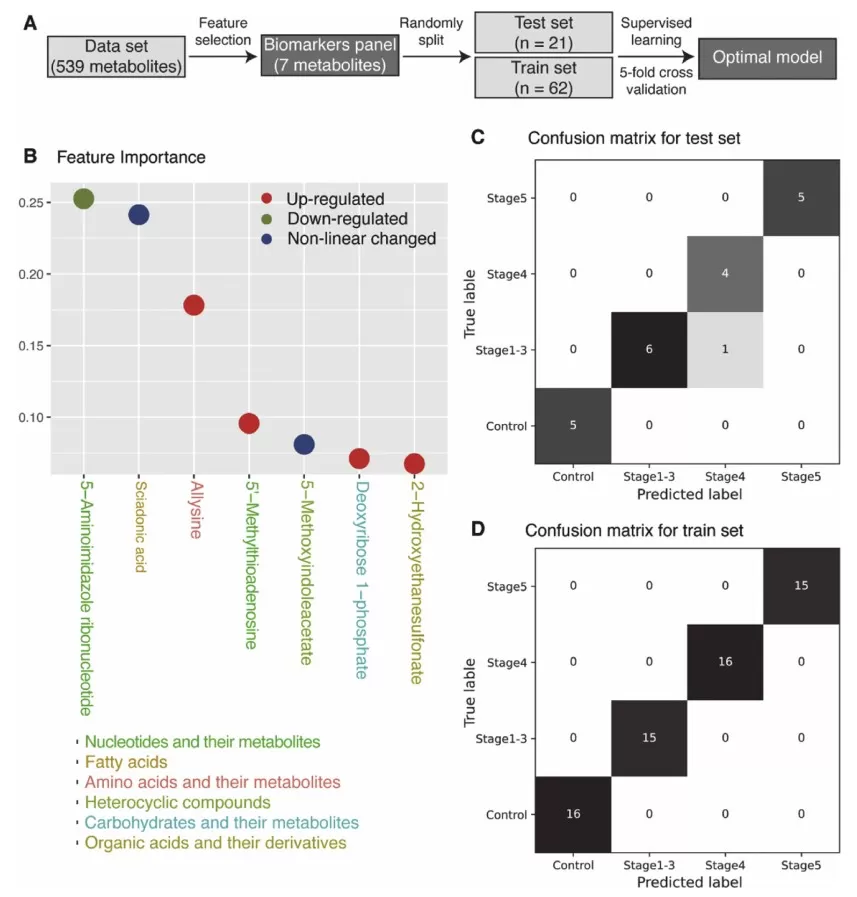
Biomarkers panel selected by machine-learning.
Comparison of Metabolite Panels and Validation
To validate the selected biomarker panel, its performance was compared with three other metabolite combinations: the top 10 up-regulated metabolites, top 10 down-regulated metabolites, and the top 10 metabolites with non-linear changes. Receiver operating characteristic (ROC) curve analysis demonstrated that the 7-metabolite panel achieved the highest classification performance, with micro-average and macro-average AUC values of 0.99. Additionally, clustering analysis using Uniform Manifold Approximation Projection (UMAP) confirmed the superior discriminatory ability of the 7-metabolite panel in distinguishing CKD patients from healthy controls.
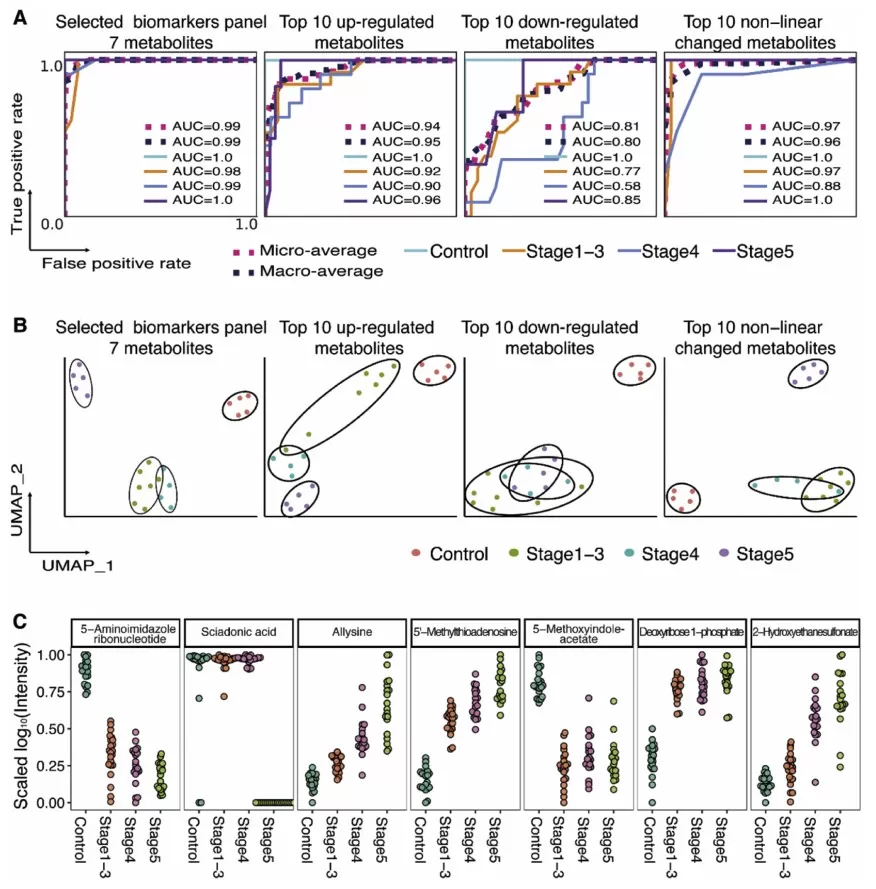
Predicting different stages of CKD using different combination of metabolites.
Metabolomics-Driven Precision Medicine: The Future of CKD Treatment
This study highlights the power of metabolomics in uncovering disease-specific metabolic signatures. The identified biomarker panel provides valuable insights into CKD progression and potential therapeutic targets. The integration of machine-learning further enhances the accuracy and clinical applicability of these findings. The identified metabolites play crucial roles in cellular and metabolic pathways that are highly relevant to kidney function. For instance, alterations in purine metabolism may indicate increased oxidative stress and mitochondrial dysfunction in CKD patients. Similarly, changes in amino acid metabolism suggest disruptions in nitrogen balance and protein catabolism. The findings align with previous studies linking metabolic dysregulation to CKD pathophysiology and highlight potential areas for further research.
The application of WT-Met in CKD research underscores the necessity of high-throughput, sensitive metabolomics platforms in disease diagnostics and monitoring. By identifying reliable metabolic markers, personalized treatment strategies can be developed to improve patient outcomes. The ability to detect metabolic shifts at early CKD stages suggests that metabolomics-based screening could be instrumental in preventive medicine.
While the study presents compelling evidence for the utility of metabolomics in CKD, further validation in larger, diverse cohorts is necessary. Additionally, investigating the mechanistic roles of these metabolites will provide deeper insights into CKD pathophysiology and potential therapeutic interventions. Future studies should explore how interventions such as dietary modifications or pharmacological treatments influence these metabolic markers.
Unlocking the Potential of Metabolomics with MetwareBio
At MetwareBio, we are dedicated to advancing metabolomics research through state-of-the-art technology and unparalleled expertise. Our widely-targeted metabolomics (WT-Met) platform offers high-throughput, sensitive, and accurate detection of metabolites, providing invaluable insights for biomedical research and clinical applications.
Our robust in-house metabolomics database, featuring over 3,000 biomedical compounds and 61,000 plant-related compounds, ensures precise and reliable metabolite identification. Utilizing cutting-edge LC-MS technology, we deliver high-sensitivity, large-scale metabolome profiling with exceptional accuracy. Our team of expert metabolomics scientists, bioinformaticians, and technical specialists brings unparalleled expertise to every project. Backed by years of experience, we offer end-to-end solutions, from experimental design and sample preparation to high-resolution data acquisition and advanced bioinformatics analysis. Committed to excellence, we ensure reproducible, high-impact results that drive scientific innovation. Furthermore, our advanced machine-learning and AI-driven analytical pipelines enable precise biomarker discovery and metabolic pathway analysis, making MetwareBio a leader in integrating metabolomics with next-generation computational tools. Our expertise spans a wide range of applications, including disease biomarker discovery, drug development, nutritional studies, and environmental metabolomics.
Partner with MetwareBio to access industry-leading metabolomics services, expert consultation, and one-stop solutions tailored to your research needs. We empower researchers and clinicians with deep insights into metabolic pathways, accelerating discoveries in precision medicine and disease diagnostics.


