Total Protein Quantification Methods: BCA vs. Bradford
Introduction to Protein Quantification Methods
Protein quantification is a crucial component of biological and medical research, measuring the concentration of proteins in specific samples. In proteomics experiments, accurate protein quantification results are vital for guiding subsequent experimental procedures and assessing the overall reliability of the experiments. Numerous methods exist for quantifying proteins, including the Kjeldahl method, biuret method, UV absorbance, Folin-Ciocalteu reagent (Lowry method), and Coomassie Brilliant Blue method (Bradford method). Although each method has its own advantages and disadvantages, the two most commonly used for total protein quantification in proteomics are the BCA and Bradford methods. Other methods tend to be less suitable for quantifying total proteins in biological samples due to inherent limitations. In this blog, we will delve into the principles, advantages, and disadvantages of the BCA and Bradford methods, aiming to enhance our application of these techniques for accurately determining protein concentrations in proteomics experiments.
BCA (Bicinchoninic Acid) Method: Principle, Advantages, and Limitations
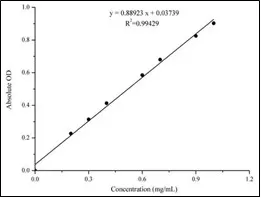 The BCA method relies on the reduction of divalent copper ions to monovalent copper ions by proteins under alkaline conditions. This reaction generates a sensitive color change, as the monovalent copper ions interact with the BCA solution. Specifically, two BCA molecules chelate one monovalent copper ion, forming a purple complex that displays strong absorbance at 562 nm. There is a robust linear relationship between absorbance and protein concentration across a wide range, allowing for the determination of protein concentration by plotting a standard curve based on the absorbance values of different standards.
The BCA method relies on the reduction of divalent copper ions to monovalent copper ions by proteins under alkaline conditions. This reaction generates a sensitive color change, as the monovalent copper ions interact with the BCA solution. Specifically, two BCA molecules chelate one monovalent copper ion, forming a purple complex that displays strong absorbance at 562 nm. There is a robust linear relationship between absorbance and protein concentration across a wide range, allowing for the determination of protein concentration by plotting a standard curve based on the absorbance values of different standards.
Advantages: The BCA method is relatively straightforward to perform, exhibits minimal dependence on protein specificity, and maintains a good linear correlation between protein concentration and absorbance. It can accurately measure protein concentrations from 50 to 1000 µg/mL, making it well-suited for assessing changes in complex protein samples. Furthermore, BCA protein quantification reagents can accommodate samples with up to 5% surfactants (detergents) and are less influenced by variations in protein composition, ensuring greater uniformity among different proteins.
Disadvantages: However, the BCA method has lower sensitivity, which can lead to inaccuracies in quantifying proteins at low concentrations. It is also susceptible to interference from certain chemicals, such as reducing agents like DTT/TCEP, and is affected by pH changes and the presence of EDTA. Additionally, variations in amino acid composition among different proteins may introduce errors in the quantification results.
 Due to the advantages and limitations of the BCA method in protein quantification, researchers have been continually improving this technique. For instance, Thermo Fisher has introduced a reduced-reactivity BCA quantification kit, which offers slight modifications to the original BCA kit. This new method eliminates the need for protein precipitation (to remove reducing agents), enabling the determination of protein concentration in samples containing disulfide reducing agents. The linear working range of this kit is from 125 to 2000 μg/mL and demonstrates good compatibility with protein samples containing up to 5 mM DTT, 35 mM BME, or 10 mM TCEP. This advancement allows for the quantification of protein concentrations in nearly all common types of samples encountered in protein research using the BCA method.
Due to the advantages and limitations of the BCA method in protein quantification, researchers have been continually improving this technique. For instance, Thermo Fisher has introduced a reduced-reactivity BCA quantification kit, which offers slight modifications to the original BCA kit. This new method eliminates the need for protein precipitation (to remove reducing agents), enabling the determination of protein concentration in samples containing disulfide reducing agents. The linear working range of this kit is from 125 to 2000 μg/mL and demonstrates good compatibility with protein samples containing up to 5 mM DTT, 35 mM BME, or 10 mM TCEP. This advancement allows for the quantification of protein concentrations in nearly all common types of samples encountered in protein research using the BCA method.
Bradford Method: Principle, Advantages, and Limitations
The Bradford method is based on the principle that Coomassie Brilliant Blue G-250 dye appears reddish-brown in its free state, with a maximum absorbance at 465 nm. When the dye binds to proteins, the color changes to blue, and the maximum absorbance shifts to 595 nm. The absorbance of the protein-dye complex at 595 nm is proportional to the protein concentration within a specific range, allowing for protein quantification through a standard curve.
Advantages:
- Protein-dye binding reaches equilibrium in about 2 minutes, enabling rapid concentration detection.
- The complex remains stable at room temperature for up to 1 hour, facilitating data recording and analysis of multiple samples.
- The method shows some tolerance to specific sample components, allowing for the quantification of protein samples containing reducing agents, withstanding up to 1 M β-mercaptoethanol and 5 mM dithiothreitol.
Disadvantages:
- Variability in arginine and aromatic amino acid content among different proteins can lead to significant deviations in concentration measurements.
- The presence of detergents, such as SDS and Triton X-100, can cause substantial quantification errors.
- The linearity of the standard curve is limited; beyond a certain concentration range, deviations become pronounced, potentially compromising the accuracy of results.
To address the need for rapid protein quantification while maintaining compatibility with detergents, researchers have developed improved Bradford protein quantification kits. For  instance, Thermo Fisher offers a detergent-compatible Bradford assay kit. This kit operates similarly to the traditional Bradford method, with the Coomassie dye binding to proteins in acidic conditions, leading to an immediate shift in absorbance from 465 nm to 595 nm, along with a color change from green to blue. The entire assay can be completed in just 10 minutes.
instance, Thermo Fisher offers a detergent-compatible Bradford assay kit. This kit operates similarly to the traditional Bradford method, with the Coomassie dye binding to proteins in acidic conditions, leading to an immediate shift in absorbance from 465 nm to 595 nm, along with a color change from green to blue. The entire assay can be completed in just 10 minutes.
Choosing the Right Protein Quantification Method for Your Research
In summary, when selecting an appropriate protein quantification method for proteomics, it is essential to consider the specific experimental requirements, sample characteristics, and available equipment. Each quantification method has its advantages and suitable applications. For cases demanding high sensitivity and a wide concentration range, along with precise quantification accuracy, the BCA method is an excellent choice. Conversely, for rapid and straightforward quantification analysis, the Bradford method is more suitable.
Next-Generation Omics Solutions:
Proteomics & Metabolomics
Ready to get started? Submit your inquiry or contact us at support-global@metwarebio.com.


