Atmospheric Pressure Chemical Ionization (APCI): Principles, Advances, and Diverse Applications
Atmospheric Pressure Chemical Ionization (APCI) stands out as a powerful ionization technique widely adopted in mass spectrometry, particularly advantageous for analyzing low to moderately polar chemical compounds. Since its inception, APCI has increasingly gained recognition due to its unique ionization mechanism, enabling it to effectively handle substances ranging from pharmaceuticals and environmental pollutants to complex petrochemical mixtures. This article systematically introduces the fundamental principles behind APCI, outlines recent technological advancements, and highlights its extensive applications across various scientific fields.
Fundamental Principles of APCI
The essence of APCI lies in its capability to generate stable molecular ions through gas-phase ion-molecule reactions at atmospheric pressure. Central to this process is a corona discharge needle, which applies a high voltage (typically around 3 kV) to produce a reactive, non-thermal plasma rich in energetic species like hydroxyl radicals (HO·), atomic oxygen radicals (O·), and positive ions (O₂⁺). These reactive species initially interact with solvent molecules such as water vapor or nitrogen to form primary ions like hydronium ions (H₃O⁺) or nitrogen cations (N₂⁺). The subsequent gas-phase reactions between these primary ions and analyte molecules typically occur through proton transfer, hydride abstraction, or charge exchange, resulting in the formation of characteristic ions ([M+H]⁺ or [M-H]⁻), crucial for accurate molecular identification.
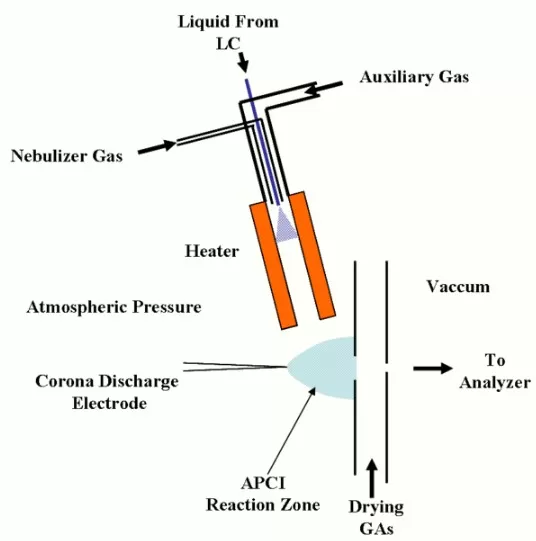
Working principles of APCI
Compared to Electrospray Ionization (ESI), APCI demonstrates particular suitability for compounds with lower polarity. While ESI requires analytes to exhibit surface activity or inherent ionization capabilities, APCI does not rely on these characteristics, making it the preferred choice for analyzing nonpolar and moderately polar compounds, such as polycyclic aromatic hydrocarbons (PAHs) and saturated hydrocarbons.
Advantages and Limitations of APCI
APCI demonstrates several notable advantages, making it highly beneficial for various analytical applications. Firstly, its efficiency in ionizing low-polarity compounds, such as linear saturated hydrocarbons (LSHs), is remarkable, as it frequently generates stable [M-H]⁻ ions essential for accurate molecular characterization. Additionally, APCI integrates seamlessly with liquid chromatography (LC), effectively handling high-flow-rate separations (1-2 mL/min), thereby enhancing analytical throughput and operational efficiency. Another critical advantage of APCI is its lower susceptibility to matrix effects compared to Electrospray Ionization (ESI), providing superior analytical reliability in complex biological matrices such as blood and plant tissues, significantly improving accuracy and precision in bioanalytical assays.
However, APCI also faces certain limitations that restrict its universal applicability. A major challenge arises from unintended oxidation reactions occurring within the ion source. The presence of highly reactive oxygen species in the corona discharge plasma can lead to the formation of undesired oxidized ions ([M+O]⁺), complicating quantitative analyses and interpretation. Furthermore, APCI demonstrates relatively lower sensitivity for strongly polar or thermally unstable analytes, which tend to ionize better through alternative methods such as electrospray ionization (ESI). This limits APCI's effectiveness for certain highly polar or thermally fragile molecules, necessitating careful consideration in method selection for comprehensive analysis.
Technological Advancements and Optimizations of APCI
Recent developments in APCI technology have significantly broadened its analytical capabilities and improved performance. A typical APCI source includes critical components such as a corona discharge needle, aerosol nebulizer, heated capillary tube, and a gas-flow control system. Each of these components plays a role in optimizing ionization efficiency and minimizing undesired side reactions.
Hybrid ionization methods have emerged as promising advancements, integrating APCI with complementary techniques like vacuum ultraviolet (VUV) and low-temperature plasma (LTP) to expand the range of ionizable compounds and reduce fragmentation. Additionally, closed-source designs that control the humidity and oxygen concentration within the ion source have effectively minimized unintended oxidation reactions, enhancing reproducibility and accuracy.
Further innovation involves dual-mode ionization sources, which simultaneously integrate APCI and electrospray ionization (ESI). This dual capability allows simultaneous analysis of both polar and nonpolar analytes, significantly increasing versatility and analytical throughput.
Diverse and Expanding Applications of APCI
APCI has found extensive applications across various scientific fields, notably in pharmaceuticals, environmental sciences, petrochemicals, and metabolomics.
In pharmaceutical analysis, APCI-MS/MS is highly effective for quantifying drugs and metabolites. A notable example is the quantification of Eldecalcitol, an active vitamin D analog, which has achieved impressive sensitivity levels (pg/mL) even within complex biological matrices. Additionally, APCI coupled with high-resolution mass spectrometry (HRMS) effectively distinguishes structural isomers of PAHs metabolites, enhancing specificity in pharmacokinetic studies.
APCI also demonstrates exceptional potential in environmental sciences, specifically in detecting persistent organic pollutants (POPs). Compared to traditional electron ionization (EI), APCI offers significantly lower detection limits for semi-volatile organic compounds and pesticides, positioning itself as an essential analytical tool for environmental monitoring.
In the petrochemical sector, APCI is uniquely advantageous in analyzing complex hydrocarbon mixtures, such as those found in petroleum distillates and waxes. Its capability to generate stable negative ions ([M-H]⁻) enables precise characterization of alkyl chain lengths and molecular structures through controlled collision-induced dissociation (CID).
Additionally, APCI has made significant strides in metabolomics and mass spectrometry imaging (MSI). Combined with laser ablation (LA) techniques, APCI facilitates the spatial mapping of metabolites like vitamins and amino acids, providing insights into biochemical processes at micrometer resolutions.
Challenges and Future Directions of APCI
Despite significant progress, APCI continues to face certain challenges. Variability across instrument manufacturers can impede method transfer and reproducibility, necessitating standardized approaches and universally accepted evaluation criteria. Moreover, managing in-source oxidation reactions remains critical for achieving high accuracy in quantitative analyses.
Looking ahead, technological integration and artificial intelligence hold promise for addressing these challenges. Intelligent ion sources featuring real-time feedback loops and sensor-based optimization represent a promising direction, capable of dynamically adjusting to varying analytical conditions to improve reproducibility and precision. The increasing use of machine learning algorithms to predict ionization behavior and optimize experimental conditions will further streamline the application of APCI, especially in high-throughput environments.
Moreover, the future likely holds an increased emphasis on hybrid and multimodal ionization sources, combining APCI with complementary techniques such as atmospheric pressure photoionization (APPI) and dielectric barrier discharge ionization (DBDI). Such integration will significantly enhance analytical versatility, enabling APCI to become a fundamental tool across diverse scientific and industrial sectors.
Reference
[1] Samarasinghe, Ishira., Pavlov, Julius., & Attygalle, Athula B. (2025). Unexpected Artifact Formation in Mass Spectrometric Analysis of Aniline under Atmospheric-Pressure Chemical Ionization. Journal of the American Society for Mass Spectrometry.
[2] Molnar, Brian T., & Shelley, Jacob T. (2020). MODERN PLASMA-BASED DESORPTION/IONIZATION: FROM ATOMS AND MOLECULES TO CHEMICAL SYNTHESIS. Mass spectrometry reviews, 40(5).
[3] Manheim, Jeremy M., Milton, Jacob R., Zhang, Y., & Kenttämaa, Hilkka I. (2020). Fragmentation of Saturated Hydrocarbons upon Atmospheric Pressure Chemical Ionization Is Caused by Proton-Transfer Reactions. Analytical chemistry, 92(13), 8883-8892.
[4] Lipok, Christian., Uteschil, Florian., & Schmitz, Oliver J. (2020). Development of an Atmospheric Pressure Chemical Ionization Interface for GC-MS. Molecules (Basel, Switzerland), 25(14).
[5] Brown, Lindsay P., Seaton, Wesley B., Powers, Joshua B., Campagna, Shawn R., & Campagna, Shawn R. (2024). Discovery of Combustion-Like in-Source Oxidation of Linear Saturated Hydrocarbons Using GC-APCI-HRMS. Journal of mass spectrometry: JMS.
[6] Brecht, Dominik., Uteschil, Florian., & Schmitz, Oliver J. (2020). Development of a fast-switching dual (ESI/APCI) ionization source for liquid chromatography/mass spectrometry. Rapid communications in mass spectrometry: RCM, 34(17), e8845.
[7] Rebane, Riin., Kruve, Anneli., Liig&, Jaanus., Liig&, Piia., & Gornischeff, Artur. (2019). Ionization efficiency ladders as tools for choosing ionization mode and solvent in liquid chromatography/mass spectrometry. Rapid communications in mass spectrometry: RCM, 33(23), 1834-1843.
[8] Huang, De-Yi., Huang, De-Yi., Wang, Meng-Jiy., Wu, Jih-Jen., & Chen, Yu-Chie. (2021). Ionization of Volatile Organics and Nonvolatile Biomolecules Directly from a Titanium Slab for Mass Spectrometric Analysis. Molecules (Basel, Switzerland), 26(22).
[9] Strmeň, Timotej., Strmeň, Timotej., Vrkoslav, Vladimír., Bosáková, Zuzana., & Cvačka, Josef. (2020). Atmospheric pressure chemical ionization mass spectrometry at low flow rates: Importance of ion source housing. Rapid communications in mass spectrometry: RCM, 34(10), e8722.


