Metabolomics for Infectious Diseases And Immune System
Infectious diseases can happen quickly. The 2019 global pandemic of COVID-19 showed that multi-omics examination and rapid pharmaceutical development are needed to combat infectious diseases. Furthermore, different people can show different symptoms of the same disease. One of the key aspects is the understanding of the metabolic effects after infection and during drug discovery, as metabolites and lipids often provide a readout during illness. Metabolomics and Lipidomics are the primary technologies to probe metabolic changes in these situations, as seen by applications in Ebola virus disease (Eisfeld et al. Cell Host Microbe 2017, Kyle et al. PNAS 2019.), HBV (Lan et al. Metabolites 2022), and HIV (Sperk et al. J. Virol 2021), COVID-19 (Wu et al. National Science Review 2020, ). The detected metabolites can be further studied for biomarkers discovery and treatment strategy development (Figure 1).
Figure 1. Rahman et al. Editorial: Metabolomics in Infectious Diseases. Front. Genet. 2022
Case Study: Plasma Metabolomic And Lipidomic Alterations Associated With COVID-19 (Wu et al., National Science Review 2020)
During the early outbreak of COVID-19, Metware Biotechnology was at the forefront of trying to understand the biology of SARS-CoV-2 and the pathogenesis of the disease. To understand the physiology and pathology of the disease, we profiled the metabolome and the lipidome of 34 patients with either mild, severe, or fatal outcomes. Blood samples from 10 healthy volunteers were used as the control group. In total, 431 metabolites and 698 lipids were identified and quantified.
Comparing the metabolites from patients with fatal outcomes with those from healthy controls, and metabolites from patients with severe or mild outcomes with those from healthy controls, we see that patients with severe or mild outcomes have fewer differential metabolites, and nearly all show down-regulation (Figure 2).
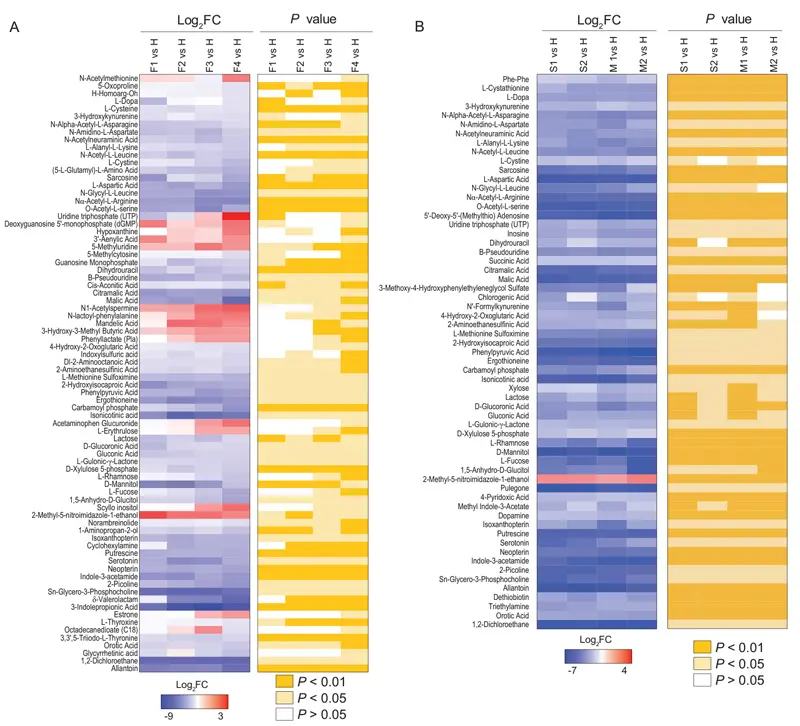
Figure 2
Result Of Metabolic Effects For Infectious Diseases
A prominent signature observed among patients with fatal outcomes was an acute reduction of metabolites in the plasma. Some of these metabolites were malic acid, aspartic acid, carbamoyl phosphate, D-xylulose 5-phosphate, guanosine monophosphate, and dihydrouracil (Figure 3). We postulate that deficiencies in malic acid and aspartate are caused, at least in part, by SARS-CoV-2 replication hijacking nucleic acids from host cells, because the TCA cycle and aspartate would be preferential for purine and pyrimidine nucleotide biosynthesis.
The combined five plasma metabolite panel discriminated the fatal group from mild group (Fig. 4B), severe group from the mild group (Fig. 4C), and fatal group from the severe group (Fig. 4D) in ROC analyses. The combined five plasma metabolites could be a useful panel for COVID-19 diagnosis.
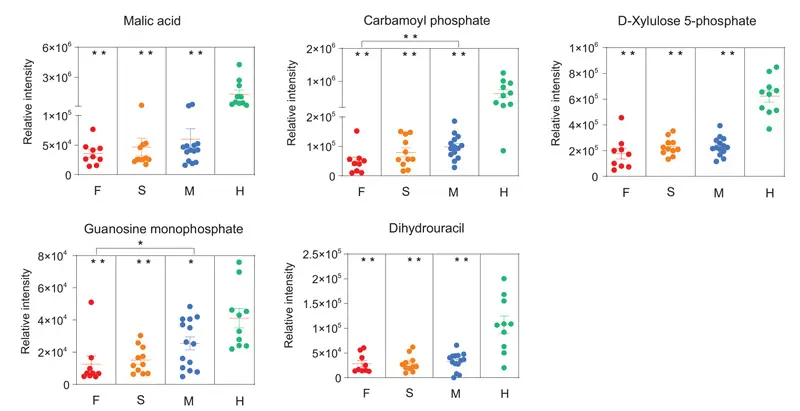
Figure 3
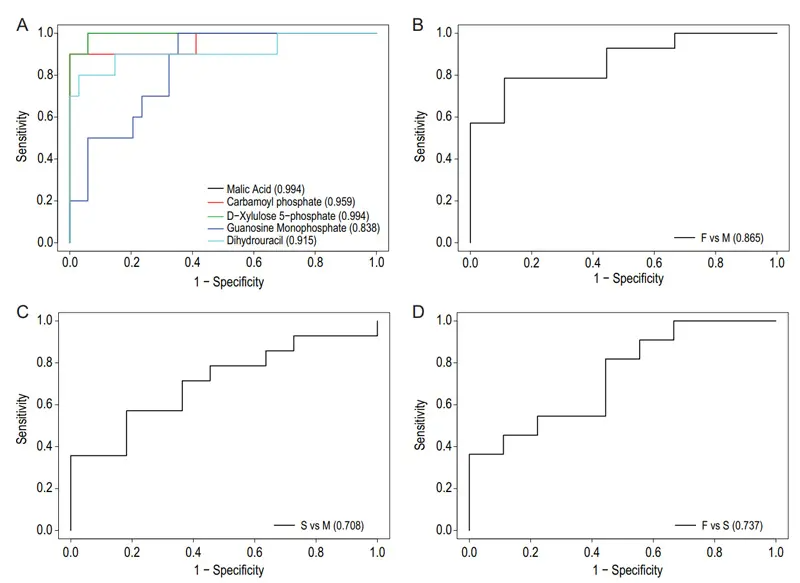
Figure 4


